Relationship Between Equilibrium Constant K, Reaction Quotient Q And Gibbs Energy G
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Equilibrium Fundamentals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning, class! Today, we will delve into the concepts of equilibrium, the equilibrium constant K, and the reaction quotient Q. Can anyone remind me what we mean by chemical equilibrium?

I think it means when the rates of the forward and reverse reactions are equal?

Exactly! In this state, the concentrations of reactants and products remain constant over time. Now, the equilibrium constant K provides a quantitative measure of this balance. Who can tell me how we express K mathematically?

K is expressed as the concentration of products divided by the concentration of reactants, raised to the power of their coefficients.

Great! So if we have a reaction such as aA + bB ⇌ cC + dD, we can write K as K = [C]^c [D]^d / [A]^a [B]^b. What happens to K if we change the concentration of reactants or products?

K remains constant, as long as the temperature is unchanged.

Correct! Now let's discuss the reaction quotient Q. How does Q relate to K?

Q has the same form as K, but it uses the current concentrations of reactants and products.

Exactly! Q provides insight into the position of the equilibrium. If Q < K, the reaction will favor the formation of products. Now, let's do a quick recap: if Q = K, where are we?

We are at equilibrium!
Gibbs Energy and Its Relation to Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Fantastic! Now, let’s discuss Gibbs energy. Why is it important when looking at chemical equilibria?

Gibbs energy helps us determine if a reaction is spontaneous?

Exactly! The change in Gibbs energy (∆G) indicates spontaneity. If ∆G < 0, the reaction is spontaneous in the forward direction, and if ∆G > 0, it favors the reverse reaction. Can someone recall the relationship between Gibbs energy and our previous segments?

The equation ∆G = ∆G° + RT ln Q! This shows how Gibbs energy changes with different concentrations.

That's right! And at equilibrium, when ∆G = 0, we can conclude that ∆G° = -RT ln K. Why is this significant?

It means that we can predict the behavior of reactions as we change conditions!

Absolutely! Manipulating concentrations, temperature, and pressure can help drive reactions to favor products or reactants. To summarize: the equilibrium is a dynamic balance characterized by K, and we can monitor shifts using Q and Gibbs energy. Well done, everyone!
Application of Gibbs Energy in Predicting Reaction Behavior
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand these relationships, how can we apply this to real-world situations in chemistry or even biology?

In metabolic pathways, reactions need to occur in a certain order and were spontaneous adjustments made?

Exactly! Enzymes can lower the activation energy and impact Gibbs energy, facilitating reactions in metabolic orchestration. In industry, how is this valuable?

Manufacturers need to optimize conditions—like temperature and pressure—to increase product yield!

Exactly! Understanding K, Q, and ∆G allows chemists to design better processes for reactions. Any final thoughts?

The concept of equilibrium ensures that we find a balance, not just in chemistry but in nature too!

Absolutely! It shows the beautiful interconnected nature of chemical systems. Excellent engagement today!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The relationship between K, Q, and Gibbs energy explains how chemical systems maintain equilibrium. K indicates the ratio of product and reactant concentrations at equilibrium, while Q shows the current ratio. Changes in these ratios influence Gibbs energy, which indicates the spontaneity of reactions, helping to predict the behavior of a chemical system under varying conditions.
Detailed
In chemical reactions, equilibrium is a dynamic state where the rate of the forward reaction equals the rate of the reverse reaction, leading to constant concentrations of reactants and products. The equilibrium constant (K) quantifies this balance, while the reaction quotient (Q) compares the current ratio of products to reactants. When Q equals K, the system is at equilibrium. The Gibbs free energy (G) is a thermodynamic quantity that indicates the spontaneity of a reaction; a negative change in G (
∆G) suggests a spontaneous reaction favoring the products, while a positive ∆G indicates a reaction likely to proceed in reverse. Key relationships include ∆G = ∆G° + RT ln Q, and at equilibrium ∆G = 0, leading to the fundamental equation: ∆G° = -RT ln K. Understanding these relationships aids chemists in manipulating conditions to achieve desired reaction pathways.
Youtube Videos



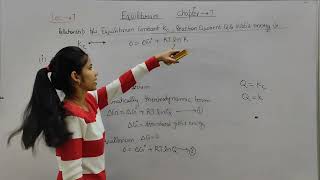




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Gibbs Energy and Equilibrium
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The value of Kc for a reaction does not depend on the rate of the reaction. However, as you have studied in Unit 5, it is directly related to the thermodynamics of the reaction and particularly, to the change in Gibbs energy, ∆G. If,
• ∆G is negative, then the reaction is spontaneous and proceeds in the forward direction.
• ∆G is positive, then the reaction is considered non-spontaneous. Instead, as reverse reaction would have a negative ∆G, the products of the forward reaction shall be converted to the reactants.
• ∆G = 0 indicates that the reaction has achieved equilibrium; at this point, there is no longer any free energy left to drive the reaction.
Detailed Explanation
Gibbs energy (∆G) is a crucial concept in understanding chemical reactions and their spontaneity. If ∆G is negative, the forward reaction is favorable, meaning the products are formed spontaneously. If ∆G is positive, the reaction needs an input of energy to proceed, indicating it is non-spontaneous. At equilibrium, ∆G equals zero, indicating that the rates of the forward and reverse reactions are equal, and no net change occurs in the concentrations of reactants and products.
Examples & Analogies
Think of Gibbs energy like a hill when you're on a hike. If you're going downhill (negative ∆G), it’s easy and spontaneous—like a reaction that occurs without needing extra energy. If you're going uphill (positive ∆G), you need to exert effort (input energy) to make progress, similar to a reaction that doesn’t happen spontaneously. When you're at the top with no more elevation to gain or lose (∆G = 0), you’re at equilibrium—balanced and stable.
Mathematical Relationship of Gibbs Energy and Equilibrium Constant
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A mathematical expression of this thermodynamic view of equilibrium can be described by the following equation:
∆G = ∆G + RT lnQ (6.21)
where, G is standard Gibbs energy. At equilibrium, when ∆G = 0 and Q = Kc, the equation (6.21) becomes, ∆G = ∆G + RT ln K = 0
∆G = –RT ln K (6.22)
This leads to the conclusion that at equilibrium, the standard Gibbs energy is related to the equilibrium constant, K, by the logarithmic function.
Detailed Explanation
This section introduces a key relationship in chemical thermodynamics. The equation shows how the Gibbs energy changes as a reaction progresses towards equilibrium. At equilibrium, the change in Gibbs energy (∆G) is zero, which means the standard Gibbs energy (∆G) relates directly to the equilibrium constant (K). This implies that a reaction with a lower energy barrier (more negative ∆G) is more likely to proceed in the forward direction, leading to a higher value of K.
Examples & Analogies
Imagine running a race downhill (forward reaction) versus uphill (reverse reaction). If you pick a good route (lower energy path), you can run faster (higher K). In terms of energy, if your route is well-planned, your chance of success (reaction proceeding spontaneously) improves dramatically, mirroring how a negative Gibbs energy leads to a high equilibrium constant.
Implications of Gibbs Energy
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If ∆G is negative, the reaction is spontaneous and proceeds in the forward direction. If ∆G is positive, the reaction is considered non-spontaneous. Instead, as reverse reaction would have a negative ∆G, the products of the forward reaction shall be converted to the reactants. If ∆G equals zero, the system has reached equilibrium.
Detailed Explanation
The Gibbs energy indicates whether a reaction will occur spontaneously. A negative ∆G means that the reaction is energetically favorable and will proceed to completion under standard conditions. Conversely, a positive ∆G indicates that the forward reaction is not favorable; it will not proceed without an external energy source. At equilibrium, there is a balance between the reactants and products, represented by ∆G = 0, implying no net change will occur in the concentrations of the species involved.
Examples & Analogies
Think of baking bread. The ingredients (reactants) need to combine to create something delicious (products). If your recipe is good (negative ∆G), the bread will rise and bake just right without extra effort (spontaneous). If you've got a stale recipe (positive ∆G), you'll struggle to make good bread until you find ways like adding yeast (providing energy) to make it rise again. When your bread is perfectly baked (equilibrium), it will stay soft, not rising or falling further.
Key Concepts
-
Equilibrium Constant (K): The ratio of concentrations of products to reactants at equilibrium.
-
Reaction Quotient (Q): The ratio of concentrations of products to reactants at any point in time.
-
Gibbs Free Energy (G): Indicates the spontaneity of a reaction based on the system's conditions.
Examples & Applications
In a reaction yielding products A and B from reactants C and D, if K = 5, products (A and B) are favored at equilibrium.
If Q = 3 and K = 5 for the same reaction, the reaction will shift to produce more products until Q = K.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
At equilibrium, K and Q will flow, reflect their ratios, let spontaneity show.
Stories
Imagine a seesaw at play, when products and reactants balance, they find their way.
Memory Tools
KQG: 'K and Q fight for the throne, which one will rise? The free energy alone!'
Acronyms
KQG
Keep Quiet.Gravity
representing K
Q
and Gibbs' energy dynamics.
Flash Cards
Glossary
- Equilibrium Constant (K)
A number that reflects the ratio of concentrations of products to reactants at equilibrium for a given reaction at a specific temperature.
- Reaction Quotient (Q)
The ratio of concentrations of products to reactants at any point in time, used to predict the direction of reaction shifts.
- Gibbs Free Energy (G)
A thermodynamic potential that measures the maximum reversible work obtainable from a thermodynamic system.
Reference links
Supplementary resources to enhance your learning experience.
