Bohr’s Model for Hydrogen Atom
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Overview of Bohr’s Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're delving into Niels Bohr’s Model of the hydrogen atom. Can anyone tell me what the main features of this model might be?

Is it about fixed paths for electrons around the nucleus?

Exactly! Electrons move in fixed orbits or stationary states around the nucleus. What's remarkable is that these orbits correspond to specific energy levels.

So, how do the electrons move between these orbits?

Great question! An electron can absorb energy to jump to a higher orbit or release energy to fall back to a lower orbit. This energy change leads to the emission of light, which we observe as spectral lines.

And those spectral lines are what make each element unique, right?

Yes! By analyzing the spectral lines, we can learn a lot about an element's atomic structure. Let's keep exploring Bohr’s quantization principle.
Bohr’s Postulates
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Bohr had several crucial postulates. The first is that only certain orbits are allowed for electrons. Can anyone summarize what this means?

It means that not all paths are allowed—only specific ones based on quantized energy levels.

Right! Quantization of energy means these paths correspond to distinct energy states. The second postulate is about angular momentum being quantized. What does that involve?

It means the angular momentum of electrons in these orbits is restricted to integral multiples of h divided by 2π.

Exactly, and this concept was key to explaining why electrons don’t spiral into the nucleus. It provides stability. Lastly, can you recall how energy transitions are represented mathematically?

The frequency of the radiation absorbed or emitted corresponds to the energy difference between the higher and lower states?

Precisely! And this leads us beautifully into the next topic: understanding hydrogen’s emission lines.
Limitations of Bohr’s Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

While Bohr’s Model was revolutionary, it had its limitations. Can you identify why?

It only works well for hydrogen and doesn’t apply to multi-electron atoms.

Exactly! It fails to explain their spectra and assumes well-defined orbits, which contradicts the Heisenberg uncertainty principle.

So, it was a stepping stone towards a better understanding through quantum mechanics?

Yes! Bohr’s work paved the way for the quantum mechanical model, which incorporates both particle and wave aspects of electrons.

And that model addresses the uncertainties?

Absolutely! We'll explore that model and its profound implications next class.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section details Bohr's Model of the hydrogen atom, which introduced quantized energy states for electrons revolving in fixed orbits around the nucleus. It explains how energy transitions between these states produce specific spectral lines, enhancing the understanding of atomic structure and spectral phenomena.
Detailed
In 1913, Niels Bohr proposed a model to accurately describe the hydrogen atom's structure and its spectral lines. According to Bohr's postulates, electrons orbit the nucleus in fixed paths called orbits or stationary states, each with a quantized energy level. An electron can only occupy certain allowed orbits, and energy is absorbed or emitted when it moves between these states. Bohr derived the expression for the electron's angular momentum in these orbits, revealing that it takes on discrete values—integral multiples of h/2π. This model successfully rationalized the hydrogen spectrum but failed for multi-electron systems, highlighting its limitations. Despite drawbacks, Bohr's approach set the foundation for later developments in quantum mechanics.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Bohr’s Model
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Neils Bohr (1913) was the first to explain quantitatively the general features of the structure of hydrogen atom and its spectrum. He used Planck’s concept of quantisation of energy. Though the theory is not the modern quantum mechanics, it can still be used to rationalize many points in the atomic structure and spectra.
Detailed Explanation
Bohr introduced his model of the hydrogen atom in 1913, building on the ideas of quantization introduced by Planck. While it does not reflect all aspects of modern quantum mechanics, it provides a foundation for understanding atomic structure and emission spectra. Bohr's approach primarily focuses on the energy levels of electrons in hydrogen.
Examples & Analogies
Think of the hydrogen atom like a musical instrument, where the allowed energies correspond to specific musical notes that can be played. Just like a musician cannot play any note they want, an electron in a hydrogen atom can only occupy certain energy levels.
Postulates of Bohr’s Model
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Bohr’s model for hydrogen atom is based on the following postulates:
i) The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states or allowed energy states. These orbits are arranged concentrically around the nucleus.
ii) The energy of an electron in the orbit does not change with time. However, the electron will move from a lower stationary state to a higher stationary state when required amount of energy is absorbed by the electron or energy is emitted when electron moves from higher stationary state to lower stationary state.
iii) The frequency of radiation absorbed or emitted when transition occurs between two stationary states that differ in energy by ∆E, is given by Bohr’s frequency rule.
iv) The angular momentum of an electron is quantised.
Detailed Explanation
Bohr's model relies on several key postulates. Firstly, electrons travel in stable, fixed orbits without radiating energy. Secondly, energy changes occur only when an electron jumps between these orbits. Thirdly, the frequency of emitted or absorbed radiation corresponds to the energy difference between the orbits. Finally, the angular momentum of an electron in an orbit is quantized, meaning it can only take on certain discrete values.
Examples & Analogies
Imagine a staircase, where each step represents a different energy level. An electron can only stand on the steps (orbits) and needs to gain or lose energy (climb up or down) to move from one step to another. Just like you can't stand between steps, an electron can't exist between allowable energy states.
Calculating Energy Levels
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Bohr calculated the energy of electron in various orbits and for each orbit predicted the distance between the electron and nucleus. According to Bohr’s theory for hydrogen atom, the stationary states for electron are numbered n = 1,2,3.......... These integral numbers are known as Principal quantum numbers.
Detailed Explanation
Bohr's calculations indicate that the energy of an electron in an orbit can be expressed using a specific formula, which depends on the principal quantum number 'n'. The lower the value of 'n', the closer the electron is to the nucleus and the lower its energy. For instance, the first orbit (n=1) has the lowest energy, while higher values of 'n' correspond to higher energy states and larger atomic radii.
Examples & Analogies
Consider the energy levels as the floors of a building. The ground floor (n=1) is the most stable and closest to the ground (nucleus), while the higher floors (n=2, n=3, etc.) are progressively further away and less stable. Just like it takes energy to move up in a building, an electron requires energy to transition to a higher orbit.
Explanation of Hydrogen’s Line Spectrum
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
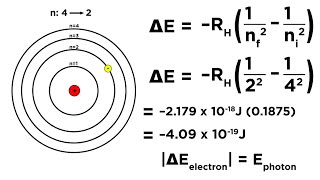
Line spectrum observed in case of hydrogen atom can be explained quantitatively using Bohr’s model. According to Bohr’s assumptions, radiation (energy) is absorbed if the electron moves from the orbit of smaller Principal quantum number to the orbit of higher Principal quantum number, while radiation is emitted if the electron moves from higher to lower orbit.
Detailed Explanation
Bohr's model allows for a clear explanation of the hydrogen atom's line spectrum. When an electron absorbs energy, it can jump to a higher energy state. Conversely, when it falls back to a lower state, it emits a photon of light with a specific frequency. The resulting spectrum consists of discrete lines corresponding to the wavelengths of the emitted light, which is unique to each element.
Examples & Analogies
Think of a playground slide. When a child slides down (moves from a higher to lower energy state), they have potential energy and convert it to kinetic energy, which can be seen as them moving quickly (emitting light). If they climb up the slide (absorb energy), they gain potential energy, but they can only reach specific heights (energy levels) defined by the slide's design.
Key Concepts
-
Bohr's Model: Revolutionized atomic theory by introducing quantization of electron orbits.
-
Quantization: Electrons can only occupy certain energy levels.
-
Angular Momentum: Defined by Bohr’s model as quantized for stable orbits.
-
Spectral Lines: Unique emissions corresponding to transitions between quantized energy levels.
Examples & Applications
When an electron transitions from the n=2 to n=1 level in hydrogen, it emits a photon of light.
The unique spectral line of hydrogen visible as the Balmer series results from electrons dropping to the second energy level.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Electrons spin, but not in a whirl, Fixed in their orbits, all around they swirl.
Stories
Imagine electrons like planets, moving in their fixed paths around the nucleus sun, absorbing and emitting light as they play.
Memory Tools
Remember: BANG - Bohr angular momentum, 'n' and 'g', for quantized paths.
Acronyms
QUAD - Quantized Unstable AuDible orbits
Bohr's model defined discrete energy levels.
Flash Cards
Glossary
- Bohr's Model
A model of the hydrogen atom proposing that electrons orbit the nucleus in specific, quantized orbits.
- Quantization
The concept that energy levels are discrete rather than continuous.
- Angular Momentum
The product of the electron's mass and velocity, which in Bohr's model is quantized.
- Spectral Lines
Lines in a spectrum corresponding to specific wavelengths of light emitted by electrons transitioning between energy levels.
Reference links
Supplementary resources to enhance your learning experience.
