Discovery of Protons and Neutrons
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Discovery of Canal Rays
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're diving into the exciting discoveries in atomic theory. Who remembers what canal rays are?

Aren't they those rays that come from the cathode in a tube?

Exactly! Canal rays reveal positive particles. Can anyone tell me what makes them different from cathode rays?

Cathode rays are made of electrons, which are negatively charged, right?

Correct! Now, what can we say about the mass of canal ray particles?

Their mass varies depending on the gas used, unlike cathode rays.

Great point! This leads us to the concept of the proton, the positively charged particle identified from hydrogen. Let's keep that in mind.
Characterization of Protons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss protons in detail. What are the unique characteristics of protons?

They have a positive charge and a specific mass, right?

Exactly! The mass of a proton is approximately 1.6726 x 10^-27 kg. And what about neutrons? Who discovered them?

James Chadwick discovered neutrons by bombarding beryllium with alpha particles!

Correct, Student_1! Neutrons are neutral and slightly heavier than protons. Now, anyone remember why the discovery of neutrons was crucial?

Because it showed that the nucleus could be stable without having just protons?

Absolutely! Neutrons help provide stability in the nucleus, and their discovery answered many questions regarding atomic integrity.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the experiments that led to the discovery of protons and neutrons as essential constituents of atoms. It describes how canal rays revealed the existence of positively charged particles and details their characteristics, leading to the identification of protons and neutrons, alongside the historical context of atomic theory.
Detailed
Detailed Summary
The rich diversity of chemical behavior among elements is primarily due to the differences in the structure of their atoms. Atoms, once thought indivisible by early philosophers, were shown to be made up of subatomic particles: electrons, protons, and neutrons. The section discusses the discoveries made towards the end of the nineteenth century regarding these particles.
Discovery of Protons and Neutrons
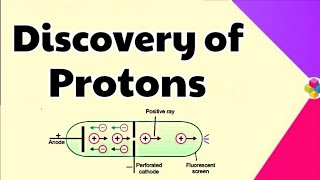
- Canal Rays: Experiments conducted with modified cathode ray tubes revealed canal rays that carried positively charged particles.
- Characteristics of Canal Rays:
- The mass of positively charged particles varies depending on the gas present in the tube.
- The charge-to-mass ratio of these particles is gas dependent.
- Some particles carry multiple units of the fundamental charge.
- Their behavior in an electric or magnetic field is opposite to that of electrons.
- Proton: The lightest positive ion observed was from hydrogen, identified as the proton in 1919.
- Neutron: The discovery of the neutron, an electrically neutral particle, followed with Chadwick's 1932 experiment involving beryllium and alpha particles. Neutrons have a mass slightly greater than that of protons.
The identification of these particles has profound implications in understanding atomic structure and behavior, answering questions about atomic stability and reactivity.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Canal Rays
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Electrical discharge carried out in the modified cathode ray tube led to the discovery of canal rays carrying positively charged particles.
Detailed Explanation
Canal rays were discovered when scientists conducted experiments using modified cathode ray tubes. Unlike cathode rays, which are negatively charged, canal rays consist of positively charged particles. This discovery was crucial in identifying the existence of protons, as canal rays are identified as positively charged gaseous ions.
Examples & Analogies
Imagine you have a magnet and you notice that one side attracts metal while the other side repels it. Canal rays act like the attracting side of the magnet, highlighting the presence of positively charged particles in atoms.
Characteristics of Canal Rays
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The characteristics of these positively charged particles are listed below:
(i) Unlike cathode rays, mass of positively charged particles depends upon the nature of gas present in the cathode ray tube. These are simply the positively charged gaseous ions.
(ii) The charge to mass ratio of the particles depends on the gas from which these originate.
(iii) Some of the positively charged particles carry a multiple of the fundamental unit of electrical charge.
(iv) The behaviour of these particles in the magnetic or electrical field is opposite to that observed for electron or cathode rays.
Detailed Explanation
The positively charged particles, known as protons, display unique characteristics compared to electrons (which make up cathode rays). The mass of these protons varies depending on the type of gas used in the cathode ray tube experiment. Additionally, some protons have a charge that is a multiple of the basic charge unit, and they behave differently than electrons when subject to magnetic or electrical fields.
Examples & Analogies
Think of different types of balls; a soccer ball may weigh different amounts when inflated with different levels of air. Similarly, the mass and behavior of protons can vary based on the gases used in the experiments.
Discovery of Protons
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The smallest and lightest positive ion was obtained from hydrogen and was called proton. This positively charged particle was characterised in 1919.
Detailed Explanation
The proton was identified as the smallest and lightest positively charged ion, and its discovery was a significant milestone in understanding atomic structure. This identification, finalized in 1919, confirmed that hydrogen consists of one proton, thus establishing protons as fundamental constituents of atomic nuclei.
Examples & Analogies
Consider a Lego block set where one special block represents a proton. Just as every building starts with a single foundational block, every hydrogen atom starts with one proton as its basic unit.
Discovery of Neutrons
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Later, a need was felt for the presence of electrically neutral particle as one of the constituents of atom. These particles were discovered by Chadwick (1932) by bombarding a thin sheet of beryllium by α-particles. When electrically neutral particles having a mass slightly greater than that of protons were emitted. He named these particles as neutrons.
Detailed Explanation
The discovery of neutrons was prompted by the understanding that atomic structure needed an electrically neutral particle to balance the positive charges of protons. Chadwick's 1932 experiments revealed neutrons as neutral particles slightly heavier than protons, which added stability to atomic nuclei by preventing protons from repelling each other due to their like charges.
Examples & Analogies
Imagine a seesaw where one side is heavier due to several people balancing on it. Neutrons act like extra weight that keeps protons (the lighter side) from moving apart, ultimately stabilizing the atomic structure.
Summary of Discoveries
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thus, we can conclude that electrons are basic constituents of all the atoms.
Detailed Explanation
In summary, various experiments conducted led to the understanding of atoms being made up of fundamental particles: electrons, protons, and neutrons. This discovery reshaped the early atomic theory, which depicted atoms as indivisible, by introducing a more complex structure of atomic components.
Examples & Analogies
Think of atoms as buildings; while the outside might look like a single structure, if you look closer, you'll find many essential components like windows, doors, and rooms, analogous to how atoms consist of multiple fundamental particles.
Key Concepts
-
Subatomic Particles: Building blocks of atoms including protons, neutrons, and electrons.
-
Canal Rays: Positively charged particles discovered in cathode ray experiments.
-
Proton Characteristics: Positively charged, found in nucleus, essential for atomic identity.
-
Neutron Discovery: Important for stability in atomic structure.
Examples & Applications
Canal rays were first introduced by experiments showing their deflection in electric and magnetic fields, proving they were positively charged.
The mass of a neutron is approximately equal to that of a proton but has no charge, illustrating how neutrons help stabilize the nucleus.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Protons are positive, neutrons are neutral, together they form an atomic jewel.
Stories
Imagine a tiny universe where positive protons and neutral neutrons dance around a nucleus, creating the building blocks of everything around us.
Memory Tools
P for Positive Proton, N for Neutron, both make the nucleus an atomic throne.
Acronyms
C for Canal rays, P for Protons, N for Neutrons - Remembering the tiny players in atomic structure.
Flash Cards
Glossary
- Proton
A positively charged subatomic particle found in the nucleus of an atom.
- Neutron
An electrically neutral subatomic particle present in the nucleus of an atom, contributing to atomic mass.
- Canal rays
Positively charged rays observed in cathode ray tubes, consisting of positively charged particles.
- Chargetomass ratio
A measurement that represents the charge per unit mass of a particle, critical for characterizing subatomic particles.
Reference links
Supplementary resources to enhance your learning experience.
