Dual Behaviour of Matter
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Dual Behaviour
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will explore the concept of dual behaviour of matter. Can anyone tell me what we mean by matter having a dual nature?

Does it mean that particles like electrons can behave both like particles and waves?

Exactly! This duality expresses that particles can exhibit properties of both waves and particles. For example, light behaves as a stream of photons and also exhibits wavelike behaviour in phenomena like interference and diffraction.

How did scientists determine that matter behaves this way?

Great question! The photoelectric effect observed by Einstein was a key experiment. When light strikes a metal, it can eject electrons, indicating that light behaves like a particle. He explained it using the concept of photons—particles of light.

So, does this mean all matter exists in both forms?

Yes. Louis de Broglie's hypothesis states that all matter has wave properties, leading to the famous equation \( λ = \frac{h}{p} \). This helps us understand the behaviour of particles at the quantum level.

To summarize, dual behaviour is pivotal for quantum mechanics. It allows us to better explain atomic and molecular interactions. Let's prepare for more complex discussions in the next session!
De Broglie's Hypothesis
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In our last session, we briefly mentioned de Broglie's hypothesis. Why is it so crucial?

It suggests that electrons and similar particles can have wavelengths.

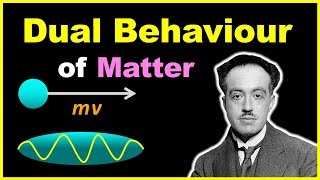
Exactly! De Broglie proposed that every moving particle has an associated wavelength given by \( λ = \frac{h}{mv} \), where \( h \) is Planck's constant, \( m \) is mass, and \( v \) is velocity. This concept revolutionized our understanding of particles.

Can we see this behaviour in real experiments?

Yes! Electron diffraction experiments confirm that electrons exhibit wave-like behaviour. When electrons pass through a double-slit, they create an interference pattern, similar to light waves.

So, does this mean that all matter can be described in wave terms?

Indeed! For most macroscopic objects, the associated wavelengths are negligible, but for subatomic particles, they are significant. This concept underpins modern quantum mechanics.

In summary, de Broglie's hypothesis is foundational in understanding the quantum realm. It shows that particles like electrons exhibit behaviours we typically associate with waves.
Implications of Dual Behaviour
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Given the dual behaviour of matter, how has our perception of atomic structure changed?

I think it allows scientists to view electrons not just as particles but as wavefunctions.

Correct! This perspective led to the development of the quantum mechanical model. It explains the probabilistic nature of electron positions.

So we can’t say exactly where an electron is?

Precisely! The Heisenberg Uncertainty Principle states we can't know both the position and momentum of a particle simultaneously. This uncertainty is central to quantum mechanics.

It sounds like we have to think in terms of probabilities.

Exactly! The quantum mechanical model incorporates wave-like properties through wave functions that give us the probability densities of finding electrons in different locations.

To conclude, the dual nature of matter has implications for atomic theory, leading to the understanding that atoms cannot be viewed in classical terms alone. Instead, quantum mechanics provides a more accurate description of micro-level phenomena.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the dual behaviour of matter, emphasizing the wave-particle duality exhibited by electrons and photons. It highlights how these concepts form the basis for modern quantum mechanics, which describes the electronic structure of atoms and their interactions.
Detailed
Dual Behaviour of Matter
The concept of 'dual behaviour of matter' refers to the principle that subatomic particles, such as electrons and photons, exhibit both particle-like and wave-like properties. This duality is essential in understanding the behavior of these particles under various conditions.
Historical Context
Until the early 20th century, classical physics could not fully explain phenomena such as the photoelectric effect, where light can eject electrons from a metal surface. Albert Einstein's work on the photoelectric effect demonstrated that light could be thought of as consisting of particles (photons), which led to the acceptance of particle-wave duality.
De Broglie's Hypothesis
In 1924, Louis de Broglie proposed that if light could behave as a particle, then matter, such as electrons, could also exhibit wave-like characteristics. De Broglie introduced the equation \
\[ λ = \frac{h}{p} \]\, where \( λ \) is the wavelength, \( h \) is Planck's constant, and \( p \) is momentum. This groundbreaking idea suggested that particles have wavelengths associated with their movement, leading to significant advancements in fields like quantum mechanics.
Significance of Wave-Particle Duality
The wave-particle duality is foundational in quantum mechanics, influencing theories and models that deal with the electronic structures of atoms. The Schrödinger equation, which describes how the quantum state of a physical system changes over time, integrates this wave-function concept and helps determine the probability densities of finding electrons in particular locations around the nucleus.
Conclusion
Understanding the dual nature of matter not only facilitates a deeper grasp of subatomic particles' behavior but also lays the groundwork for developing modern theories of atomic and molecular structure.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Dual Behaviour
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The French physicist, de Broglie, in 1924 proposed that matter, like radiation, should also exhibit dual behaviour i.e., both particle and wavelike properties.
Detailed Explanation
In 1924, de Broglie suggested that just like light can behave both as a wave (which can spread out and interfere) and as a particle (like photons), matter, such as electrons, can also exhibit both particle and wave characteristics. This idea was revolutionary because it changed how scientists viewed the very nature of matter and energy.
Examples & Analogies
Imagine you're at a concert where a band is playing. You can see the band (particles), but you can also feel the sound wave flowing through the air (waves). Similarly, electrons can be seen as tiny particles moving around, but their path can also behave like waves, creating patterns like ripples on a pond.
de Broglie's Relation
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This means that just as the photon has momentum as well as wavelength, electrons should also have momentum as well as wavelength.
Detailed Explanation
de Broglie's equation is a mathematical representation that correlates the wavelength (λ) of a particle to its momentum (p). It suggests that anything with mass, including electrons, should possess wave-like behavior characterized by a wavelength. The formula is given as λ = h/p, where h is Planck’s constant. This principle implies that the physical mass of particles like electrons is not only a measure of their mass but also relates to how they spread out in space.
Examples & Analogies
Picture waves in a calm lake. If you throw a stone into the water, the ripples (waves) move outward. When thinking of an electron, you can imagine it as a small stone thrown into the ocean, creating waves. These waves are like the behavior of electrons as they move around the nucleus of an atom.
Experimental Confirmation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
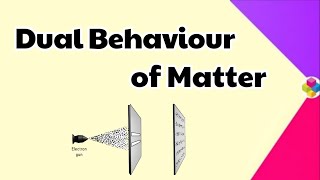
de Broglie’s prediction was confirmed experimentally when it was found that an electron beam undergoes diffraction, a phenomenon characteristic of waves.
Detailed Explanation
The concept of wave-particle duality was strongly supported by experiments where beams of electrons displayed diffraction patterns. Just like light waves can bend and interfere with one another when passing through slits, electrons can also do the same, suggesting that they possess wave-like characteristics. This was one of the first demonstrations that particles could display behaviors typical of waves.
Examples & Analogies
Think of how light creates patterns when it shines through a set of narrow slits. Similarly, if you throw a handful of sand through a sieve, some grains may get caught while others pass through, creating patterns on the ground. In a similar way, when electrons are narrowed through slits, they create unique patterns that prove their wave-like behavior.
Electron Microscopes
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This fact has been put to use in making an electron microscope, which is based on the wavelike behaviour of electrons just as an ordinary microscope utilises the wave nature of light.
Detailed Explanation
Electron microscopes leverage the wave properties of electrons to achieve much higher resolutions compared to optical microscopes that use light waves. Because electrons have much shorter wavelengths than visible light, they can reveal much finer details of the objects being observed. This immense power of electron microscopy allows scientists to explore and analyze structures at the molecular and atomic levels.
Examples & Analogies
Imagine trying to read tiny print using a magnifying glass—this is like using a regular light microscope. Now, picture using a super high-end camera that sees even finer details, like the textures of a painting up close. This advanced camera represents the electron microscope, revealing details that we otherwise could not see with regular light.
Key Concepts
-
Wave-Particle Duality: Refers to the concept that matter exhibits both wave-like and particle-like properties.
-
Heisenberg Uncertainty Principle: Suggests that the precise position and momentum of an electron cannot be measured simultaneously.
-
De Broglie's Hypothesis: Proposes that matter has wave properties, giving rise to wave functions that describe electrons.
Examples & Applications
The photoelectric effect demonstrates that photons behave like particles, as they can eject electrons from a surface.
Electron diffraction experiments show that electrons exhibit wave-like behavior, creating interference patterns.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the quantum realm, waves dance and swirl, particles express, a magical whirl.
Stories
Imagine walking on a beach where waves crash. Every step you take sends ripples into the water. Just like light, which sends particles moving. Yet both the waves and steps create unique patterns.
Memory Tools
WAVE: W is for Wave Particles, A is for All Matter, V is for Velocity and E is for Energy, all these aspects reflect dual nature.
Acronyms
DWAP
Dual Wave-Particle Theory.
Flash Cards
Glossary
- Dual Behaviour
The property of matter exhibiting both wave-like and particle-like characteristics.
- Photons
Particles of light that exhibit both particle-like and wave-like properties.
- Wave Function
A mathematical function that describes the wave-like state of a particle, providing information about potential locations of that particle.
- Heisenberg Uncertainty Principle
A principle stating that it is impossible to know both the exact position and momentum of a particle simultaneously.
Reference links
Supplementary resources to enhance your learning experience.
