Filling of Orbitals in Atom
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding the Aufbau Principle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start with the Aufbau principle, which helps us understand how electrons fill the orbitals in an atom. Can anyone tell me what the term 'Aufbau' means?

I think it means 'building up' in German, right?

Exactly! It indicates that electrons occupy orbitals starting from the lowest energy available. Can someone give me an example of this principle?

Hydrogen would fill its 1s orbital first before moving to any higher orbitals.

Correct! That's a perfect application of the principle. We typically write the filling order of the orbitals as a sequence, which we can remember. Who can tell me the order?

1s, 2s, 2p, 3s, 3p, 4s, 3d, etc.

Great! That's the filling order we use. Now, remember, while this order helps, exceptions can occur. Let's explore that further with the Pauli exclusion principle.
Pauli Exclusion Principle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The Pauli exclusion principle tells us that no two electrons can have the same set of quantum numbers. How does that affect orbital filling?

It means that each orbital can hold a maximum of two electrons, and they must have opposite spins.

Exactly! This prevents overcrowding in orbitals. Can anyone explain what we mean by 'quantum numbers'?

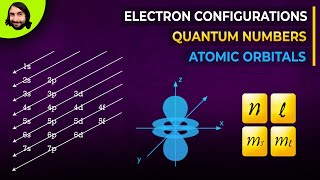
Quantum numbers include values like n, l, and ml, which define the energy, shape, and orientation of orbitals.

Well done! So remember, the Pauli exclusion principle is crucial for correctly filling electrons without duplicating quantum states. Let's now combine our knowledge with Hund's rule.
Hund's Rule of Maximum Multiplicity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss Hund's rule. It states that electrons will fill degenerate orbitals singly and with the same spin direction before pairing up. Why do you think this is important?

I think it’s to minimize electron repulsion in orbitals of the same energy.

Absolutely! Lower repulsion means greater stability. Can we see an example demonstrating Hund's rule?

In the case of p orbitals, when there are three available p orbitals, each will get one electron first before any get a second.

Exactly! That leads us to a more stable electronic configuration. Can someone summarize what we’ve learned today about filling orbitals?

We learned that orbitals fill in order of energy, no two electrons can be in the same state, and that pairing occurs after each orbital is singly filled.

Perfect summary! Understanding these principles will help you predict the electronic configurations of various elements.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Electrons occupy orbitals in a specific order based on their energy levels, following the Aufbau principle, Pauli exclusion principle, and Hund’s rule. Understanding this process is crucial for predicting the electronic configurations of different atoms.
Detailed
The filling of electrons into the orbitals of atoms follows a specific order governed by three fundamental rules and principles:
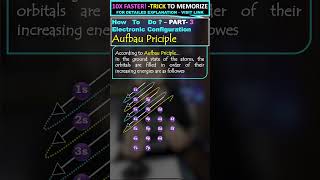
- Aufbau Principle: Electrons fill the lowest energy orbitals first before moving to higher energy orbitals. The general order of filling is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, etc.
- Pauli Exclusion Principle: No two electrons in the same atom can have the identical set of four quantum numbers, which implies that an orbital can hold a maximum of two electrons, which must have opposite spins.
- Hund's Rule: When electrons occupy orbitals of the same energy, they will fill them singly before pairing up in orbitals. This arrangement minimizes electron-electron repulsion and increases stability.
Understanding these principles helps explain the electronic structure of atoms, which is vital in predicting chemical behavior and bonding patterns.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Aufbau Principle
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The word ‘aufbau’ in German means ‘building up’. The building up of orbitals means the filling up of orbitals with electrons. The principle states: in the ground state of the atoms, the orbitals are filled in order of their increasing energies. In other words, electrons first occupy the lowest energy orbital available to them and enter into higher energy orbitals only after the lower energy orbitals are filled. As you have learnt above, energy of a given orbital depends upon effective nuclear charge and different type of orbitals are affected to different extents. Thus, there is no single ordering of energies of orbitals which will be universally correct for all atoms. However, the following order of energies of the orbitals is extremely useful: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 4f, 5d, 6p, 7s...
Detailed Explanation
The Aufbau Principle provides guidance on how electrons are arranged in an atom, based on their energy levels. Electrons fill the lowest available energy level first before moving to higher levels. This means that an atom will fill its orbitals from 1s to 2s to 2p, and so on, according to their energy levels. The specific sequence may vary for different elements, but this principle serves as a foundational guideline.
Examples & Analogies
Think of filling seats in a theater. The best seats are filled first (the lowest energy orbitals), and the less desirable seats (higher energy orbitals) are filled only after the best ones are taken. This way, everyone gets the best experience available! Similarly, atoms arrange their electrons in the order of energy preferences, filling the most stable configurations first.
Pauli Exclusion Principle
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The number of electrons to be filled in various orbitals is restricted by the exclusion principle given by the Austrian scientist Wolfgang Pauli (1926). According to this principle: no two electrons in an atom can have the same set of four quantum numbers. Pauli exclusion principle can also be stated as: 'only two electrons may exist in the same orbital and these electrons must have opposite spin.' This means that the two electrons can have the same value of three quantum numbers n, l, and ml, but must have the opposite spin quantum number...
Detailed Explanation
The Pauli Exclusion Principle states that even within the same orbital, a maximum of two electrons can exist, and they must spin in opposite directions (up and down). This prevents any two electrons from having identical quantum states, thereby ensuring that the configuration of electrons around a nucleus remains unique. Because of this principle, we can determine the maximum number of electrons that any given subshell can hold.
Examples & Analogies
Imagine a shared bedroom where only two people can sleep in a bed. To make it fair, they must take turns sleeping, so one person sleeps on their side while the other sleeps in the opposite direction. This mimics how electrons behave within an orbital—they can occupy the same space, but must 'rotate' in opposite directions to accommodate each other's presence.
Hund’s Rule of Maximum Multiplicity
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This rule deals with the filling of electrons into the orbitals belonging to the same subshell (that is, orbitals of equal energy, called degenerate orbitals). It states: pairing of electrons in the orbitals belonging to the same subshell (p, d or f) does not take place until each orbital belonging to that subshell has got one electron each i.e., it is singly occupied...
Detailed Explanation
Hund’s Rule emphasizes that when electrons occupy orbitals of the same energy level, they will first fill each orbital singly, each with their spins parallel, before they start to pair up in orbitals. This arrangement minimizes electron-electron repulsion and lowers the energy of the atom, making it more stable. This principle accounts for the specific patterns observed in electron configurations.
Examples & Analogies
Imagine a group of friends trying to sit on a row of swings at a park. Instead of multiple friends crowding onto one swing, they each choose an empty swing first (single occupancy), which makes it more enjoyable. Once all swings are occupied by one person, then they can start sharing swings (pairing up). This approach achieves a better balance and social experience—just like Hund’s Rule helps stabilize electron arrangements!
Electronic Configuration of Atoms
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The distribution of electrons into orbitals of an atom is called its electronic configuration. If one keeps in mind the basic rules which govern the filling of different atomic orbitals, the electronic configurations of different atoms can be written very easily...
Detailed Explanation
The electronic configuration of an atom is a representation of the number of electrons distributed among its orbitals. Understanding how to write electronic configurations requires knowledge of the aforementioned principles, including Aufbau, Pauli, and Hund’s Rule, which dictate how electrons occupy different orbitals. This configuration gives insights into the chemical properties and reactivity of elements.
Examples & Analogies
Think of electronic configurations as organizing books on library shelves. Each shelf can only hold a certain number of books (electrons), and each book has its unique place according to specific cataloging rules (Aufbau, Pauli, Hund’s Rule). When you know how to categorize the books based on these rules, locating them becomes much easier, just as knowing electronic configurations makes predicting an atom's behavior easier in chemical reactions.
Key Concepts
-
Aufbau Principle: Electrons fill the lowest energy orbitals first.
-
Pauli Exclusion Principle: No two electrons can have the same quantum numbers.
-
Hund's Rule: Electrons fill orbitals singly before pairing up.
Examples & Applications
The electron configuration of helium (He) is 1s2, filling the 1s orbital completely.
In nitrogen (N), with 7 electrons, the configuration is 1s2 2s2 2p3, following Hund's Rule.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Electrons fill from low to high, / In orbitals they twist and fly.
Stories
Imagine a party where no two people can occupy the same seat (Pauli Exclusion) until each seat has one person (Hund's Rule).
Memory Tools
Think of 'A' for Aufbau, 'P' for Pauli, and 'H' for Hund - each helps you remember how to fill orbitals.
Acronyms
APH - Aufbau, Pauli, Hund
The order of filling electron orbitals.
Flash Cards
Glossary
- Aufbau Principle
Electrons fill orbitals starting from the lowest energy levels to higher.
- Pauli Exclusion Principle
No two electrons in an atom can have the same set of four quantum numbers.
- Hund's Rule
Electrons will fill degenerate orbitals singly before pairing occurs to minimize repulsion.
- Quantum Numbers
Values that describe the properties of atomic orbitals and the electrons in those orbitals.
- Degenerate Orbitals
Orbitals that have the same energy level.
Reference links
Supplementary resources to enhance your learning experience.
