Rutherford Nuclear Model of Atom
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Rutherford's Experiment and Observations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore Rutherford's nuclear model of the atom. Can anyone tell me what Rutherford did to discover the structure of the atom?

He used alpha particles to bombard a thin foil of gold, right?

Exactly! And what were some of the major observations he made during this experiment?

Most of the alpha particles went straight through the foil, but a few were deflected strongly.

Well put! What does this tell us about the atomic structure?

It suggests that most of the atom is empty space, but there’s something dense at the center that deflects some particles.

Correct! This led to the conclusion that atoms have a small, dense, positively charged nucleus. Remember, 'Most of the atom is empty space.' Can we take a moment to think about what the implications of these observations mean for our view of atoms?
Conclusions from Rutherford's Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Building on what we've learned, what did Rutherford conclude about where electrons are located?

He concluded that electrons orbit around the nucleus, kind of like planets orbiting the sun.

Exactly! This resembles a miniature solar system. However, what notable challenges does this model face regarding stability?

Well, I think that since electrons are charged, they should radiate energy and spiral into the nucleus.

Right again! This contradiction highlights the need for further developments in atomic theory, leading us into the Bohr model. Always remember, 'Electrons in circular orbits should spiral into the nucleus.'
Significance of Rutherford's Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

So, why is Rutherford’s model so significant in the context of atomic history?

It provided a more detailed view of atomic structure, showing that atoms have a nucleus.

That's correct! It laid the groundwork for future atomic theories. Students, what do you think was the next big step taken in atomic theory after Rutherford?

Wasn't it the Bohr model that followed it?

Absolutely! Keep in mind the phrase 'Rutherford's experiment -> nucleus discovery -> paved the way for Bohr!' This will help you remember the logical progression in atomic models.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Rutherford's model significantly advanced our understanding of atomic structure, demonstrating that the positive charge and most of an atom's mass are concentrated in a small nucleus, with electrons orbiting around it. This model provided an important foundation for subsequent atomic theories, although it also raised questions about atomic stability.
Detailed
Rutherford Nuclear Model of Atom
The Rutherford Nuclear Model of the atom emerged from experiments conducted in the early 20th century, especially the famous gold foil experiment. Ernest Rutherford, alongside his colleagues Hans Geiger and Ernest Marsden, bombarded thin gold foil with alpha particles. Their observations led to three crucial conclusions about atomic structure:
- Most of the atom is empty space: Most alpha particles passed through the foil without deflection, indicating that most of the atomic volume is empty.
- Existence of a dense, positively charged nucleus: Some alpha particles were deflected at large angles, suggesting that a tiny, dense center, or nucleus, must contain most of the atom's mass and positive charge.
- Electrons orbit the nucleus: Electrons are positioned outside the nucleus, balancing the positive charge of protons within it.
This model resembled a miniature solar system, where electrons orbit a central nucleus. Despite its advances, the Rutherford model could not explain the stability of these orbits, raising questions of atomic stability. Understanding that electrons in circular orbits should theoretically spiral into the nucleus due to electromagnetic radiation was a significant limitation, ultimately requiring further development of atomic theory into the Bohr Model and eventually quantum mechanics.
Overall, Rutherford's contributions were pivotal in laying the groundwork for modern atomic theory, leading to our current understanding of the atom's structure.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Rutherford's Scattering Experiment
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
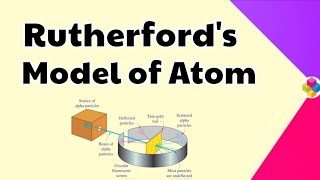
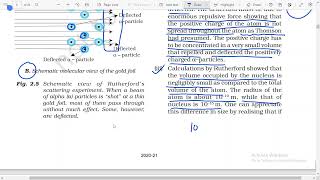
Rutherford and his students (Hans Geiger and Ernest Marsden) bombarded very thin gold foil with α-particles. Rutherford’s famous α-particle scattering experiment is depicted where a stream of high energy α–particles from a radioactive source is directed at a thin foil of gold metal. This was done to observe the deflection of particles as they passed through the foil.
Detailed Explanation
In Rutherford's experiment, alpha particles were fired at a very thin sheet of gold foil. Most alpha particles passed straight through with little or no deflection. A few particles were deflected at small angles, and an even smaller fraction (about 1 in 20,000) bounced back almost directly. This unexpected result led Rutherford to deduce that an atom is mostly empty space with a very small, dense, positively charged nucleus at its center.
Examples & Analogies
Imagine shooting marbles through a sheet of thin cardboard. Most marbles can easily pass through, but if there's a large, heavy object (like a ball) behind the cardboard, a few marbles might bounce back when they hit that heavy object. In this analogy, the marbles represent alpha particles, the cardboard represents the gold foil, and the ball represents the nucleus of the atom.
Conclusions from the Experiment
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Based on the observations from the scattering experiment, Rutherford drew the following conclusions regarding the structure of the atom: 1. Most of the atom's space is empty. 2. The positive charge is concentrated in a very small volume, termed the nucleus. 3. The nucleus contains most of the atom's mass, while electrons revolve around it.
Detailed Explanation
Rutherford concluded that because most alpha particles passed through undeflected, most of the atom must be empty space. The deflections indicated the presence of a small, dense center of positive charge (the nucleus) capable of repelling the positively charged alpha particles. He also noted that the nucleus takes up a tiny fraction of the total volume of the atom, explaining why atoms are primarily composed of empty space.
Examples & Analogies
Think of a large stadium with a tiny dot in the center. The dot represents the nucleus, while the vast empty space of the stadium symbolizes the electron cloud surrounding it. Just as spectators (electrons) move around the stadium, they do not often interact with the dot (the nucleus) because the distance is so great.
The Structure of the Rutherford Model
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
According to Rutherford’s model, the positive charge and most of the mass of the atom are concentrated in the nucleus, surrounded by electrons that move rapidly around it in orbits akin to planets around a sun.
Detailed Explanation
The Rutherford model depicts the atom as having a compact nucleus containing protons (and later determined neutrons) with electrons moving around it at a distance. This resembles a miniature solar system where the nucleus serves as the sun and the electrons as the planets. The model highlights the charges: the protons are positively charged, while the electrons are negatively charged, creating electrostatic forces of attraction that keep the electrons in orbit around the nucleus.
Examples & Analogies
This is similar to our solar system, where the sun has a large mass and the planets orbit around it. If you consider the sun as the nucleus and planets as electrons, this helps visualize how electrons are attracted to the nucleus due to its positive charge, much like how planets are attracted to the sun's gravity.
Key Concepts
-
Nuclear Model: The concept introduced by Rutherford that atoms consist of a dense nucleus surrounded by orbiting electrons.
-
Alpha Particle Scattering: A method used to probe atomic structure leading to the discovery of the nucleus.
Examples & Applications
The behavior of alpha particles in Rutherford's experiment leading to the conclusion that the atom is mostly empty space.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In every atom, there’s a core so small, protons are packed in, electrons don’t fall.
Stories
Imagine a solar system with a tiny sun—the nucleus—and fast-moving planets—electrons—whirling around it.
Memory Tools
N.E.E. - Nucleus, Electrons exist, Empty space (to remember atomic structure).
Acronyms
N.E.E - Nucleus, Electrons, Empty space.
Flash Cards
Glossary
- Nucleus
The central, dense part of an atom containing protons and neutrons.
- Alpha particles
Positively charged particles used in Rutherford's experiment to probe atomic structure.
- Electromagnetic radiation
Radiation including visible light, radio waves, and X-rays, emitted or absorbed during electron transitions.
- Electrons
Negatively charged subatomic particles that orbit the nucleus.
Reference links
Supplementary resources to enhance your learning experience.
