Structure of Atom
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Atomic Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today, we'll delve into the structure of the atom. Can anyone tell me what an atom is?

Isn't it the smallest unit of matter?

That's correct! An atom is indeed the smallest unit of matter that retains its identity. This idea has been around since ancient times. How did our understanding of atoms change over time?

I think John Dalton was the first to propose a scientific atomic theory, right?

Yes, exactly! Dalton’s atomic theory laid the foundation for modern chemistry. He proposed that atoms are indivisible and combine in specific ratios. Can anyone name a limitation of this theory?

It couldn't explain why certain materials conduct electricity or how electric charges behave.

Great point! This limitation led to the discovery of the electron by J.J. Thomson later. Let's remember that the atomic theory has evolved significantly!
Discovery of Sub-Atomic Particles
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, let’s talk about the discovery of sub-atomic particles. Who can tell me the charge of an electron?

Electrons are negatively charged!

Correct! Thomson measured the electron's charge to mass ratio. Does anyone recall what method he used?

He used a cathode ray tube!

Yes! That experiment led to groundbreaking discoveries. However, electrons are just part of the atom. When were protons discovered?

Protons were identified by Rutherford, right?

That's right! His gold foil experiment showed that there is a dense nucleus. Can anyone tell me about neutrons?

Neutrons were discovered by Chadwick in 1932.

Excellent! The discovery of neutrons was crucial in understanding atomic structure further. Keep these key figures in mind!
Models of the Atom
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss models. Who can summarize Thomson's model for us?

Thomson's model is like a 'plum pudding' where electrons are embedded in a positively charged sphere.

Great description! Now, what was the flaw in this model observed by Rutherford?

Rutherford found that most alpha particles went through, implying that most of the atom is empty space with a small nucleus.

Correct! Rutherford proposed a nuclear model. So how did Bohr improve upon it?

Bohr introduced quantized orbits for electrons!

Exactly! Bohr’s model quantifies stability. However, he also couldn’t explain multi-electron atoms. What did we learn to address these shortcomings?

The quantum mechanical model introduced wave functions!

Exactly! Understanding wave functions leads to our modern atomic theory.
Quantum Mechanical Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s delve into the quantum mechanical model. Can anyone explain what Schrödinger’s contribution was?

He proposed a mathematical description of wave functions to represent electrons!

Correct! The Schrödinger equation describes quantized energy levels. What is a key difference between Bohr and the quantum model?

The quantum model describes probabilities, not fixed orbits.

Exactly! Electrons are described by ohm's probability densities. Would anyone like to give an example of how this is used in multi-electron atoms?

The energy levels depend on both n and l quantum numbers!

Very good! And how does this relate to electron configurations?

Electrons fill up orbitals based on increasing energy according to rules like the Aufbau principle!

Perfect summary! Remember, these principles govern not only our understanding of the atom but also chemical behavior.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the historical development of atomic theory, from early philosophical concepts to the modern quantum mechanical model of the atom. It explains the discoveries of sub-atomic particles and outlines key atomic models, including those proposed by Thomson, Rutherford, and Bohr. The segment also highlights the principles governing electron configuration and atomic stability.
Detailed
Detailed Summary
The structure of an atom is central to understanding chemistry as it explains the diverse chemical behavior of elements. Initially, philosophical speculations about atoms being the indivisible units of matter were made by ancient philosophers. However, it wasn't until John Dalton in 1808 that atomic theory was formalized scientifically, establishing the atom as the fundamental building block of matter.
Dalton's atomic theory explained several chemical laws but fell short in addressing phenomena such as electric charge behavior in materials like glass. This paved the way for later experimental evidence in the late 19th and early 20th centuries, which revealed that atoms consist of three primary sub-atomic particles: electrons, protons, and neutrons.
Key experiments leading to the identification of these particles involved:
- Discovery of the Electron: Conducted by J.J. Thomson using cathode ray tubes, he discovered the presence of negatively charged electrons.
- Charge to Mass Ratio of the Electron: Thomson further measured this ratio, contributing to our understanding of the electron's properties.
- Discovery of Protons and Neutrons: Protons were identified through canal rays while neutrons were discovered by Chadwick during alpha particle bombardment experiments.
Following these discoveries, the development of atomic models began:
1. Thomson's Model proposed a 'plum pudding' arrangement where electrons were embedded within a positively charged sphere.
2. Rutherford's Model emerged from his gold foil experiment, demonstrating a dense nucleus, with electrons orbiting around it, akin to a solar system.
3. Bohr's Model improved upon Rutherford’s by quantifying electron orbits, necessitating quantized states for electrons in hydrogen.
However, Bohr's model was limited in applications to hydrogen and could not explain the complexity of multi-electron atoms. The quantum mechanical model, derived from Schrödinger's wave equation, resolved these limitations and introduced the concept of atomic orbitals defined by quantum numbers, where electron positions are described in terms of probabilities rather than fixed orbits.
Thus, modern atomic theory integrates the discoveries and models mentioned to provide a comprehensive picture of atomic structure and electron configuration, understanding how these underlie chemical reactions.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to the Structure of Atoms
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The rich diversity of chemical behaviour of different elements can be traced to the differences in the internal structure of atoms of these elements. The existence of atoms has been proposed since the time of early Indian and Greek philosophers (400 B.C.) who were of the view that atoms are the fundamental building blocks of matter. According to them, the continued subdivisions of matter would ultimately yield atoms which would not be further divisible. The word ‘atom’ has been derived from the Greek word ‘a-tomio’ which means ‘uncut-able’ or ‘non-divisible’. These earlier ideas were mere speculations and there was no way to test them experimentally. These ideas remained dormant for a very long time and were revived again by scientists in the nineteenth century.
Detailed Explanation
This chunk introduces the concept of atoms as the basic building blocks of matter. It mentions historical perspectives from ancient Indian and Greek philosophers who speculated about the indivisibility of matter, leading to the derivation of the term 'atom'. This sets the foundation for understanding how scientific inquiry has evolved over time.
Examples & Analogies
Think of an atom as a small LEGO block. Just like how LEGO blocks come together to form complex structures, different atoms combine in various ways to create everything we see around us. The idea that you can't break a LEGO block into smaller pieces without losing its identity mirrors the ancient idea of atoms being indivisible.
Discovery of Sub-atomic Particles
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
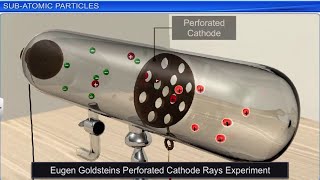
An insight into the structure of the atom was obtained from the experiments on electrical discharge through gases. Before we discuss these results we need to keep in mind a basic rule regarding the behaviour of charged particles: 'Like charges repel each other and unlike charges attract each other'.
Detailed Explanation
This chunk discusses the experimental findings that led to the discovery of sub-atomic particles, specifically through experiments involving electrical discharge in gases. It emphasizes the fundamental rule of electrostatics—that like charges repel and unlike charges attract—which is crucial for understanding atomic structure.
Examples & Analogies
Imagine you have magnets in your classroom. When you try to bring two north poles together, they push away from each other because they are like charges. However, if you bring a north pole close to a south pole, they pull towards each other because they are unlike. This interaction is similar to what happens with charged particles in an atom.
Electrons - Discovery and Properties
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In 1830, Michael Faraday showed that if electricity is passed through a solution of an electrolyte, chemical reactions occurred at the electrodes, which resulted in the liberation and deposition of matter at the electrodes. This suggested the particulate nature of electricity. In mid-1850s many scientists mainly Faraday began to study electrical discharge in partially evacuated tubes, known as cathode ray discharge tubes.
Detailed Explanation
This chunk delves into the discovery of electrons, highlighting Michael Faraday's experiments that indicated the existence of particles in electric charge. The focus on cathode ray tubes illustrates how early scientists began to uncover the nature of electricity and its relationship to atomic structure.
Examples & Analogies
Think of it like trying to understand how a light bulb works. You know it lights up when the circuit is complete, but if you open it up, you'll see the tiny particles—electrons—that create the light. Faraday's experiments were the initial steps towards revealing the 'light' in understanding atomic structure.
The Charge-to-Mass Ratio of an Electron
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In 1897, British physicist J.J. Thomson measured the ratio of electrical charge (e) to the mass of electron (me) using the cathode ray tube and applying electrical and magnetic field perpendicular to each other. Thomson argued that the amount of deviation of the particles from their path depends on the magnitude of the negative charge on the particle, the mass of the particle, and the strength of the electric or magnetic field.
Detailed Explanation
This chunk introduces J.J. Thomson's experimentation that determined the charge-to-mass ratio of electrons. The principles behind his observations are explained with a focus on how charge, mass, and field strength influence the behavior of electrons in an electric field.
Examples & Analogies
Consider a basketball and a tennis ball. If both are thrown at the same speed towards a wall with a fan blowing towards one, the lighter tennis ball will get pushed further away than the heavier basketball. Similarly, in Thomson's experiment, the charge and mass of the electrons influenced their paths through electric fields.
Discovery of Protons and Neutrons
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Electrical discharge carried out in the modified cathode ray tube led to the discovery of canal rays carrying positively charged particles. The behaviour of these particles in the magnetic or electrical field is opposite to that observed for electrons or cathode rays. The smallest and lightest positive ion was obtained from hydrogen and was called proton.
Detailed Explanation
This chunk covers the discovery of protons, highlighting how experiments with cathode rays revealed positively charged particles, which were identified as protons. It contrasts their behavior with that of electrons, linking the charge and mass properties of these sub-atomic particles.
Examples & Analogies
Think about how a magnet works. The north side of a magnet (like a proton) behaves differently when placed near another magnet compared to the south side (like an electron). This analogy helps illustrate how protons and electrons interact differently in atomic structures.
Atomic Models: Thomson and Rutherford
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thomson proposed that an atom possesses a spherical shape with positive charge uniformly distributed, while Rutherford concluded that the positive charge and most of the mass of the atom was densely concentrated in a very small region called the nucleus.
Detailed Explanation
This chunk contrasts the Thomson and Rutherford models of the atom. Thomson's model depicted a 'plum pudding' atom, while Rutherford's gold foil experiment led to the understanding that atoms have a small, dense nucleus. This evolution in thought marked significant advancement in atomic theory.
Examples & Analogies
Envision a mini solar system. Thomson's model is like a soft, squishy ball filled with raisins (electrons), while Rutherford's model resembles a hard marble (nucleus) in the center surrounded by planets (electrons) revolving around it. This analogy helps visualize how the structure of atoms began to be understood.
Key Concepts
-
Atomic Theory: Evolution of the concept of the atom.
-
Subatomic Particles: Definition and discovery of electrons, protons, and neutrons.
-
Atomic Models: From Thomson to Bohr, describing the successive improvements in the atomic model.
-
Quantum Mechanics: The transition to a probabilistic understanding of electron behavior.
Examples & Applications
Thomson's Plum Pudding Model: A representation of the atom as a uniform sphere with embedded electrons.
Rutherford's Gold Foil Experiment: Demonstrating the existence of a small, dense nucleus with the majority of the atom being empty space.
Bohr Model of Hydrogen: Describing quantized electron orbits and their energies.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Atoms come in three, protons, neutrons, electrons you see!
Stories
Imagine an orchard (the atom) where the tree (nucleus) is surrounded by buzzing bees (electrons) flying about. The tree holds the bees securely, but keeps them dancing around.
Memory Tools
Remember 'PNES': Protons, Neutrons, Electrons, Structure!
Acronyms
APPE
Atomic Theory
Protons
Particles
Electrons.
Flash Cards
Glossary
- Atom
The smallest unit of matter that retains the properties of an element.
- Electron
A negatively charged subatomic particle found in all atoms.
- Proton
A positively charged subatomic particle located in the nucleus of an atom.
- Neutron
An electrically neutral subatomic particle found in the nucleus.
- Quantum Mechanics
The branch of physics that deals with the behavior of matter and light on the atomic and subatomic scale.
- Atomic Orbital
A mathematical function that describes the wave-like behavior of an electron in an atom.
- Quantum Number
A number that quantifies the energy levels of an electron in an atom.
Reference links
Supplementary resources to enhance your learning experience.
