Drawbacks of Rutherford Model
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Rutherford Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we'll discuss the Rutherford model. This model portrays the atom as a small, dense nucleus surrounded by electrons orbiting in defined paths, much like planets around the sun.

What does this model tell us about the behavior of electrons?

Great question! It suggests that electrons occupy stable orbits around the nucleus, influenced by electrostatic forces. However, this leads to some serious questions about stability.

Why is stability a problem?

If we apply classical physics, an accelerating charged particle, like an electron in orbit, should emit energy and spiral inward, collapsing to the nucleus.

So, does that mean atoms should not exist as we know them?

Exactly! This contradiction is one of the primary drawbacks of Rutherford's model.

Does it say anything about electron energy levels?

Not at all. The Rutherford model lacks an explanation for the distribution and energy states of electrons, leading us to consider further advancements in atomic theory.
Failures in Explaining Atomic Stability
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

So, what leads to the instability in Rutherford's model?

It has to do with the emission of electromagnetic radiation, right?

Absolutely! As electrons orbit, they should lose energy and move closer to the nucleus.

Could this be why atomic structure seems more complicated?

Yes! This limitation pushed scientists to find better models, leading us towards quantum mechanics.

What was the next significant model developed to address these issues?

Neils Bohr proposed a modified model that introduced quantized electron energy levels, providing a framework for stability.
Electron Distribution and Energy Levels
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We need to break down how Rutherford's model lacks detail about electron structures.

Why is that important?

Electron distribution defines chemical behavior and stability of elements. Without understanding their arrangement, we can't predict how atoms interact.

Doesn't the Bohr model resolve this by quantizing energy levels?

Yes, precisely! However, it further reinforced the conclusion that Rutherford's model wasn't comprehensive enough.

In summary, Rutherford's model gave a starting point but proved insufficient?

Exactly! It opened the door for subsequent theories that better explained atomic structure.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
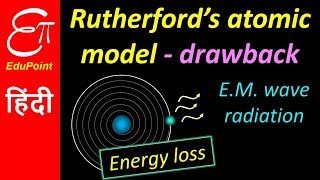
Although Rutherford's nuclear model provided insights into atomic structure, it had significant shortcomings, including the inability to account for the stability of electrons in orbits and the lack of detail regarding electron energy states and distributions.
Detailed
Drawbacks of Rutherford Model
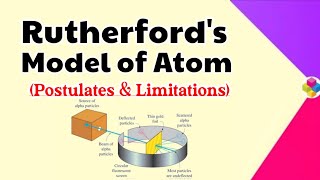
Rutherford's model of the atom presented a groundbreaking view of atomic structure, proposing that atoms consist of a dense nucleus surrounded by electrons, akin to a solar system. However, this model has several notable drawbacks:
- Stability of Atoms: According to classical mechanics, any charged particle moving in a circular orbit (like an electron) must emit electromagnetic radiation as it accelerates. This would result in a gradual loss of energy, causing the electron to spiral inward and eventually collapse into the nucleus. This prediction starkly contradicts the observed stability of atoms.
- Electron Distribution: The model does not provide a description of the distribution or energy levels of electrons around the nucleus. It lacks a framework to explain why electrons occupy specific energy states and how they are arranged in orbitals.
- Behavior of Electrons: The lack of a mechanism to explain electron arrangements and energies led to further theoretical advancements, eventually contributing to the development of quantum mechanics and the Bohr model. Bohr's model addressed some of these deficiencies by quantizing electron energy levels, thereby providing a clearer picture of stability and electron transitions.
In summary, while Rutherford's nuclear model was a pivotal step in understanding atomic structure, its fundamental limitations necessitated the evolution of atomic theory towards more comprehensive models.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Classical Mechanics and Electron Orbits
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
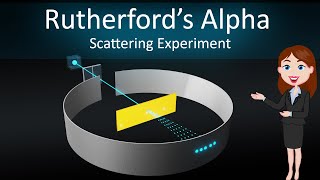
As you have learnt above, Rutherford nuclear model of an atom is like a small scale solar system with the nucleus playing the role of the massive sun and the electrons being similar to the lighter planets. When classical mechanics is applied to the solar system, it shows that the planets describe well-defined orbits around the sun.
Detailed Explanation
This chunk explains how the Rutherford model compares to a solar system, where the nucleus acts like the sun and the electrons revolve around the nucleus like planets. It highlights that according to classical mechanics, these planets (or electrons) should have clear, predictable orbits. While this analogy seems logical, it leads to issues when considering how electrons behave in reality.
Examples & Analogies
Imagine a spinning top. Just like the top spins in a predictable path, classical mechanics suggests that electrons should do the same around the nucleus. However, if you spin the top too fast or on an uneven surface, its path becomes unpredictable, which is similar to how real electrons behave.
Problems of Electron Acceleration
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
However, when a body is moving in an orbit, it undergoes acceleration even if it is moving with a constant speed in an orbit because of changing direction. So an electron in the nuclear model describing planet-like orbits is under acceleration.
Detailed Explanation
This chunk delves into the physics behind why electrons cannot simply revolve around the nucleus like planets. It states that even if an electron moves with constant speed, changing direction means it is accelerating. According to electromagnetic theory, an accelerating charge (like an electron) should emit radiation and lose energy, which would cause it to spiral into the nucleus over time.
Examples & Analogies
Think of a car going around a bend. Even if the car maintains a constant speed, it is changing direction and thus accelerating. If the car continually loses energy due to friction or obstacles, it might not keep going. Similarly, electrons would lose energy and spiral inwards if they emitted radiation due to acceleration.
Stability of Atoms
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
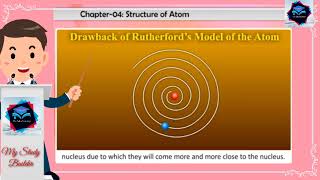
Calculations show that it should take an electron only 10–8 s to spiral into the nucleus. But this does not happen. Thus, the Rutherford model cannot explain the stability of an atom.
Detailed Explanation
This section points out a significant flaw in Rutherford's model. If classical physics were correct, electrons would quickly lose energy and crash into the nucleus in mere nanoseconds, leading to an unstable atom structure. However, atoms are stable, contrary to what this model predicts, indicating serious limitations in the Rutherford framework.
Examples & Analogies
Imagine a skateboarder on a circular track who starts to lose balance as they go around because of friction. Instead of maintaining a stable orbit, they would eventually fall off. This analogy highlights how if electrons were bound by classical mechanics, they wouldn’t remain stable in their orbits but spiral into the nucleus instead.
Electrostatic Attraction vs. Stationary Electrons
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If the motion of an electron is described on the basis of the classical mechanics and electromagnetic theory, you may ask that since the motion of electrons in orbits is leading to the instability of the atom, then why not consider electrons as stationary around the nucleus.
Detailed Explanation
This chunk suggests an alternative view: if electrons were stationary rather than orbiting, they would experience a strong electrostatic pull from the positively charged nucleus. This would cause them to collapse into the nucleus, leading to a model even less stable than Rutherford's.
Examples & Analogies
Think of a ball resting at the bottom of a bowl. It might appear stable, but a small push (the electrostatic force) would cause it to roll up the sides of the bowl and overcome the balance, leading it back towards the center where it's bound to fall, paralleling how electrons would be drawn into the nucleus.
Lack of Electron Distribution Understanding
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Another serious drawback of the Rutherford model is that it says nothing about the distribution of the electrons around the nucleus and the energies of these electrons.
Detailed Explanation
This part critiques the Rutherford model for failing to address how electrons are arranged or distributed in relation to the nucleus and how this affects their energy levels. Understanding electron configuration is crucial for predicting chemical behavior, and the Rutherford model does not provide these critical insights.
Examples & Analogies
Imagine trying to navigate a crowded room where you can't see anyone's positions or moves. A good understanding of where everyone is and how they interact is crucial for planning your actions. In the context of atoms, similar understanding of electron distribution is vital for explaining reactions and bonding.
Key Concepts
-
Inability to Explain Stability: The Rutherford model fails to describe why electrons do not spiral into the nucleus.
-
Lack of Electron Distribution Details: The model does not specify how electrons occupy energy states and levels.
-
Transition to Quantum Mechanics: Limitations led to the development of Bohr's model and quantum mechanics.
Examples & Applications
Rutherford's nuclear model illustrates the main concept of a dense nucleus and surrounding electrons.
The failure of Rutherford's model to explain atomic stability sets the stage for developments in quantum models.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In Rutherford’s model, nuclear core, with electrons that incessantly soar.
Stories
Imagine a solar system where planets are like electrons, happily orbiting their sun, the nucleus, but oh! They lose energy and fall... thus, the need for better models.
Memory Tools
Remember the 'Rutherford Rule': If your model spirals into the nucleus, it's time to renew!
Acronyms
R SPACE (Rutherford's Stability Problems and Atomic Construction Errors) to remember the flaws in the Rutherford model.
Flash Cards
Glossary
- Rutherford Model
An atomic model proposing that atoms consist of a small, dense nucleus surrounded by orbiting electrons.
- Electromagnetic Radiation
Energy emitted from charged particles when they accelerate, potentially leading to atom instability.
- Bohr Model
An improvement upon the Rutherford model, introducing quantized electron orbits to explain atomic stability.
Reference links
Supplementary resources to enhance your learning experience.
