Discovery of Sub-atomic Particles
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Atomic Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will delve into the evolution of atomic theory, beginning with the ancient ideas of atoms as uncuttable particles. Can anyone tell me what the word 'atom' means?

It means 'indivisible', right?

Exactly! But as science progressed, we discovered that atoms are not indivisible. John Dalton proposed that atoms are the smallest units of matter in 1808. What were some limitations of Dalton's theory?

It didn't explain how atoms can interact or what happens when they are charged!

Great point! This leads us to the discovery of sub-atomic particles, starting with electrons.
Discovery of Electrons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

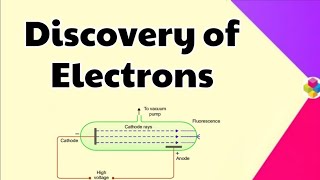
Michael Faraday's experiments suggested that electricity might be particulate. Who can describe what cathode rays are?

Cathode rays are streams of electrons emitted from the cathode.

Very well stated! Thomson's experiments led him to determine the charge-to-mass ratio of electrons to be 1.758820 x 10^11 C/kg. Why is this significant?

It helped confirm that electrons are fundamental components of all atoms!

Correct! Understanding this paved the way for modern atomic theory.
Discovery of Protons and Neutrons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

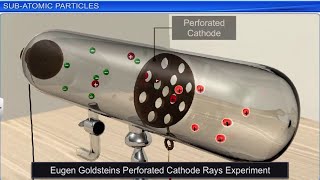
Now, let's discuss how the discovery of canal rays led to understanding protons. Can anyone summarize what canal rays are?

They are beams of positive ions that were identified in gas discharge tubes.

Perfect! In 1919, protons were designated. What about neutrons—who discovered them, and how?

Chadwick discovered neutrons by bombarding beryllium with alpha particles in 1932.

Exactly! Thus, we've moved from solid atoms to atoms filled with electrons, protons, and neutrons.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section details the evolution of atomic theory, emphasizing the pivotal experiments by key scientists like J.J. Thomson and Ernest Rutherford that revealed the existence of electrons, protons, and neutrons. It also highlights the impact of these discoveries on the development of atomic models and the understanding of chemical behavior.
Detailed
Discovery of Sub-atomic Particles
The exploration of atomic structure initiated with philosophers in ancient cultures, but the modern atomic theory began with John Dalton in the early 19th century. Dalton proposed that atoms are indivisible; however, this theory evolved significantly, particularly with the discovery of sub-atomic particles: electrons, protons, and neutrons.
Key Historical Experiments
- Discovery of the Electron:
- Michael Faraday's experiments in the 1830s with electrolytic solutions hinted at the concept of particulate electricity, leading researchers to cathode ray experiments in the mid-1850s.
- J.J. Thomson, in 1897, identified cathode rays as streams of negatively charged electrons and measured their charge-to-mass ratio, establishing the electron as a fundamental particle of all atoms.
- Discovery of the Proton and Neutron:
- The discovery of canal rays in cathode ray tubes led to the identification of positively charged particles, termed protons, in 1919. J.J. Chadwick later discovered neutrons in 1932.
Importance of Discoveries
These sub-atomic particles led to new atomic models, ultimately influencing the complexity of chemical behavior across different elements. Rutherford's model introduced the concept of a compact nucleus, rewriting the understanding that atoms consist of a small, dense core surrounded by electrons. This model was foundational for subsequent theories, paving the way for quantum mechanical models of atomic structure.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Insights from Electrical Discharge Experiments
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An insight into the structure of atom was obtained from the experiments on electrical discharge through gases. Before we discuss these results we need to keep in mind a basic rule regarding the behaviour of charged particles: 'Like charges repel each other and unlike charges attract each other'.
Detailed Explanation
The structure of an atom started to be understood through experiments that involved passing electric currents through gases. A key principle to remember is that charged particles behave in predictable ways: if two particles have the same charge (both positive or both negative), they will push away from each other. However, if their charges are different (one positive and one negative), they will attract each other. This foundational principle helps scientists interpret the behavior of particles in the atom and was essential to the developments of atomic theory.
Examples & Analogies
You can think of this as how magnets work. If you try to bring two north poles of magnets together, they repel each other, just like two positive charges. Conversely, when you bring a north and a south pole together, they pull toward each other, similar to how opposite electrical charges attract.
Discovery of the Electron
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In 1830, Michael Faraday showed that if electricity is passed through a solution of an electrolyte, chemical reactions occurred at the electrodes, which resulted in the liberation and deposition of matter at the electrodes. He formulated certain laws which you will study in Class XII. These results suggested the particulate nature of electricity.
Detailed Explanation
Michael Faraday’s experiments revealed that passing electricity through a solution causes chemical changes that produce visible effects at the electrodes. This provided early evidence that electricity is made up of particles, thus hinting at the existence of smaller components within atoms that would later be identified as electrons.
Examples & Analogies
Consider how light bulbs work. When electricity flows through a filament, it lights up, showing us that something is happening at a particle level. Similarly, when electricity passes through a solution, it shows that electric energy can create a reaction at the atomic level, leading to discoveries about the constitution of atoms.
Cathode Ray Experiments
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In mid 1850s, many scientists, mainly Faraday, began to study electrical discharge in partially evacuated tubes, known as cathode ray discharge tubes. When sufficiently high voltage is applied across the electrodes, current starts flowing through a stream of particles moving in the tube from the negative electrode (cathode) to the positive electrode (anode). These were called cathode rays or cathode ray particles.
Detailed Explanation
Scientists began using cathode ray tubes to explore the properties of electricity. They discovered that when a high voltage is applied to these tubes, particles, now known as electrons, flow from the cathode, which is negative, to the anode, which is positive. This research was crucial in identifying electrons as real, physical components of atoms.
Examples & Analogies
Imagine a water slide that has a continuous flow of water downwards. In this analogy, the water represents the particles moving from one point to another, similar to how electrons move through a cathode ray tube. Just as we can see the water flowing due to the slope of the slide, scientists saw the 'flow' of electrons when high voltage was applied.
Charge to Mass Ratio of Electron
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In 1897, British physicist J.J. Thomson measured the ratio of electrical charge (e) to the mass of electron (me) using cathode ray tube and applying electrical and magnetic field perpendicular to each other as well as to the path of electrons.
Detailed Explanation
Using cathode rays, Thomson was able to determine the charge-to-mass ratio of the electron, which provided a numeric value that indicated how much charge the electron had in comparison to its mass. This discovery helped identify the electron's fundamental properties.
Examples & Analogies
Think of the charge-to-mass ratio as comparing two different kinds of fruit in terms of weight and sweetness. By knowing both aspects, you can understand better how they behave in different situations, just like scientists sought to understand the electron by looking at its charge and mass.
Charge on the Electron
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
R.A. Millikan devised a method known as the oil drop experiment to determine the charge on the electrons. He found the charge on the electron to be – 1.6 × 10–19 C. The present accepted value of electrical charge is – 1.602176 × 10–19 C.
Detailed Explanation
Millikan's oil drop experiment involved measuring the force on tiny oil droplets that had acquired an electric charge. By balancing these forces, he could calculate the charge on the electron very accurately. This experiment provided the precise measurement of an electron's charge.
Examples & Analogies
Consider pouring a small amount of food coloring into water. If you add just the right drop, it will turn the whole glass of water to a distinct color. Similarly, Millikan's careful measurements allowed him to identify the exact charge of the electron among all the other properties it possesses.
Discovery of Protons and Neutrons
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Electrical discharge carried out in the modified cathode ray tube led to the discovery of canal rays carrying positively charged particles. Some of the positively charged particles carry a multiple of the fundamental unit of electrical charge.
Detailed Explanation
When researchers experimented with modified cathode ray tubes, they discovered rays of positively charged particles, which were later identified as protons. This showed not only that electrons exist but also that there are positively charged components within the atom as well. The exploration in this area also eventually led to the identification of the neutron as a neutral particle in the atomic nucleus.
Examples & Analogies
Think of a battery. It has both positive and negative terminals, much like how an atom contains positively charged protons and negatively charged electrons. Just as both terminals are necessary for the battery to function, both types of particles are necessary for the atom to be stable and behave in a predictable manner.
Key Concepts
-
Sub-atomic particles: Electrons, protons, and neutrons make up an atom's structure.
-
Cathode rays are a stream of electrons, leading to the discovery of the electron.
-
The charge-to-mass ratio is essential for identifying the characteristics of electrons.
-
The discovery of protons and neutrons shaped the modern atomic model.
Examples & Applications
An example of electron detection is the cathode ray tube experiment conducted by J.J. Thomson.
Rutherford's alpha scattering experiment demonstrated that the nucleus is positively charged and very small compared to the atom as a whole.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Electrons are small and negatively charged, that’s why they’re all the rage!
Stories
Once upon a time in an atom, tiny creatures called electrons danced around a central nucleus, where protons and neutrons lived, keeping perfect harmony.
Memory Tools
PEN: Protons are Positive, Electrons are Negative.
Acronyms
A Basic Atom
P.E.N – Protons
Electrons
Neutrons.
Flash Cards
Glossary
- Electron
A negatively charged subatomic particle found in all atoms.
- Proton
A positively charged subatomic particle found in the nucleus of an atom.
- Neutron
A neutral subatomic particle located in the nucleus of an atom.
- Cathode Ray
A stream of electrons observed in vacuum tubes.
- ChargetoMass Ratio (e/m)
A measurement expressing the charge of a particle per unit mass.
Reference links
Supplementary resources to enhance your learning experience.
