Wave Nature of Electromagnetic Radiation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Maxwell's Theory of Electromagnetic Waves
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss Maxwell's theory of electromagnetic waves. Can anyone tell me how these waves are formed?

Electromagnetic waves are formed by oscillating electric and magnetic fields.

Exactly! When charged particles accelerate, they create changing electric fields, which in turn produce magnetic fields.

So, these waves can travel through a vacuum as well?

Yes! That's one of the unique characteristics of electromagnetic waves—they don't require a medium to travel through. They can propagate in empty space.

What are the different types of electromagnetic radiation?

Great question! The electromagnetic spectrum includes radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays. Each type has different properties and applications.

How do we calculate the frequency or wavelength of these waves?

We can use the formula: c = νλ, where c is the speed of light. If we know one of the values, we can easily find the other. Remember, the speed of all electromagnetic waves in a vacuum is constant.

To sum up, Maxwell's work was crucial in understanding electromagnetic waves and laid the foundation for future studies in quantum theory.
Planck's Quantum Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's discuss Planck's quantum theory. Who can explain what it means to say energy is quantized?

It means energy can only be absorbed or emitted in discrete units, not in a continuous manner.

Exactly! Planck proposed that the energy of a photon is given by E = hν, where h is Planck's constant. This idea was revolutionary.

So, that means light behaves like a particle as well?

That's right! This dual nature of light is key to understanding many phenomena, like the photoelectric effect. Can anyone describe that?

It’s when light shines on a metal surface and ejects electrons only if the light has a certain frequency.

Spot on! This indicates that light must have enough energy to overcome the binding energy of the electrons in the metal, which is linked to the frequency of the light.

To conclude, Planck's theory showed us that energy transitions in atoms are quantized and paved the way to modern quantum mechanics.
Applications and Examples of Electromagnetic Radiation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s explore some practical applications of electromagnetic radiation. Can anyone give an example?

Microwaves are used for cooking food!

That’s right! Microwaves use specific wavelengths to heat water molecules in food, making cooking efficient.

What about infrared radiation?

Infrared radiation is commonly used in thermal imaging to detect heat signatures, useful in surveillance and firefighting.

And X-rays are used in medicine!

Indeed! X-rays can penetrate soft tissues, providing images of bones and other structures inside the body.

To summarize, electromagnetic radiation has diverse applications, each utilizing different types of radiation based on their unique properties.
The Significance of Electromagnetic Radiation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s discuss the significance of electromagnetic radiation in understanding atomic structure. What role does it play?

It helps us understand how electrons interact with photons.

Exactly! The absorption and emission of electromagnetic radiation by atoms result in distinct atomic spectra, which can tell us about the energy levels of electrons.

Does this mean we can identify elements using their spectra?

Precisely! Each element has a unique spectral fingerprint based on its electron configuration, aiding in identifying substances in various fields, from chemistry to astronomy.

In conclusion, electromagnetic radiation forms a cornerstone for modern physics and chemistry, enhancing our understanding of the atomic world.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the dual nature of electromagnetic radiation is examined, highlighting the historical development of its understanding through key concepts such as electromagnetic waves, the electromagnetic spectrum, and Planck's quantum theory. The insights gained from studying these waves have profound implications for atomic theory and the behavior of light in relation to electrons.
Detailed
Detailed Summary
The wave nature of electromagnetic radiation comes into focus in this section, building upon the experimental discoveries of the 19th and early 20th centuries. Physicists like James Clerk Maxwell formulated the theory of electromagnetic waves, establishing that light is not just a particle but a wave that can propagate through vacuum.
Maxwell's work demonstrated that changing electric fields produce magnetic fields and vice-versa, giving rise to electromagnetic waves that travel at the constant speed of light in a vacuum, approximately 3.0 x 10^8 m/s. This insight led to the formulation of the electromagnetic spectrum, which encompasses a range of radiation types based on frequency and wavelength.
The electromagnetic spectrum includes radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays, differing in their applications and interactions with matter. Each type of radiation is characterized by its frequency (ν) and wavelength (λ), and these two properties are related through the equation:
- c = νλ
Where c is the speed of light. Students learn how to calculate frequency and wavelength, exploring real-world examples such as radio frequencies and X-ray applications.
Planck's quantum theory introduces the idea that energy absorbed or emitted in the form of radiation is quantized, leading to the understanding of phenomena such as the photoelectric effect. This concept involves the interaction between light and electrons in atoms, providing insights into electronic structures and atomic behavior. Understanding the wave nature of electromagnetic radiation is crucial for delving deeper into atomic models and the mechanisms governing atomic spectra.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Thermal Radiation
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In the mid-nineteenth century, physicists actively studied absorption and emission of radiation by heated objects. These are called thermal radiations. It is now a well-known fact that thermal radiations consist of electromagnetic waves of various frequencies or wavelengths.
Detailed Explanation
Thermal radiation refers to the energy emitted by an object due to its temperature. When objects are heated, they emit radiation that can be detected as light or infrared heat. Physicists studied these emissions to understand their properties and found that they are made up of electromagnetic waves, which can vary in wavelength and frequency, forming the basis of the electromagnetic spectrum.
Examples & Analogies
Imagine a campfire. As the logs burn, they not only produce smoke but also emit heat that you can feel from a distance. This heat is thermal radiation, and if you could measure it, you’d find it includes a variety of wavelengths, some of which are visible light that we see as the glow of the fire.
Maxwell's Contribution
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The first active study of thermal radiation laws occurred in the 1850s, and the theory of electromagnetic waves and the emission of such waves by accelerating charged particles was developed in the early 1870s by James Clerk Maxwell, which was experimentally confirmed later by Heinrich Hertz.
Detailed Explanation
James Clerk Maxwell's work unified the understanding of electricity and magnetism, leading to the theory that when charged particles accelerate, they emit electromagnetic waves. Hertz's experiments verified this by producing and detecting these waves, laying the groundwork for our understanding of radio waves and other forms of electromagnetic radiation.
Examples & Analogies
Think of Maxwell's theory like a stone thrown into a pond—just as the stone creates ripples that spread out in circles, accelerating charged particles create electromagnetic waves that travel through space, forming the basis of technologies like radio and wi-fi.
Nature of Electromagnetic Waves
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
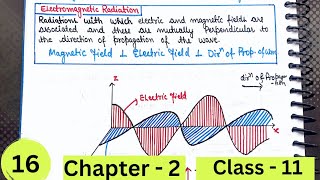
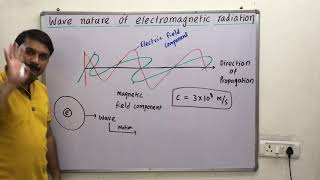
Maxwell was the first to reveal that light waves are associated with oscillating electric and magnetic fields. The oscillating electric and magnetic fields produced by oscillating charged particles are perpendicular to each other and both are perpendicular to the direction of propagation of the wave.
Detailed Explanation
Maxwell discovered that light is not just a wave traveling through space; it consists of oscillating electric and magnetic fields that regenerate each other. This means as one field changes, it creates the other, and they propagate together through space. The perpendicular nature of these fields is fundamental to how light and other forms of electromagnetic radiation behave.
Examples & Analogies
Imagine waving a rope up and down; the wave travels along the length of the rope while the peaks and valleys (oscillations) are perpendicular to the direction the wave is moving. Similarly, in electromagnetic waves, the electric field oscillates in one direction, while the magnetic field oscillates perpendicular to it, creating a 'ripple' effect through space.
Properties of Electromagnetic Radiation
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Unlike sound waves or waves produced in water, electromagnetic waves do not require medium and can move in vacuum. It is now well established that there are many types of electromagnetic radiations, which differ from one another in wavelength (or frequency). These constitute what is called electromagnetic spectrum.
Detailed Explanation
Electromagnetic waves can travel through empty space, unlike sound waves that require a medium like air or water. The electromagnetic spectrum encompasses all these waves, categorized by their wavelengths. Different types of electromagnetic radiation, from radio waves to gamma rays, differ in their frequency and wavelength but all travel at the speed of light in a vacuum.
Examples & Analogies
Think of the electromagnetic spectrum like a rainbow. Each color represents a different wavelength, just like different types of light, radio waves, and x-rays represent different parts of the spectrum. In the same way light can be split into colors, the spectrum shows all the forms of electromagnetic radiation that fill our universe.
Understanding Wave Properties
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Different regions of the spectrum are identified by different names. Some examples are: radio frequency region around 10^6 Hz, used for broadcasting; microwave region around 10^10 Hz used for radar; infrared region around 10^13 Hz used for heating; ultraviolet region around 10^16 Hz a component of sun’s radiation. The small portion around 10^15 Hz is what is ordinarily called visible light.
Detailed Explanation
The electromagnetic spectrum is divided into regions based on frequency and wavelength. Each type of wave has different applications. For instance, radio waves are used for communication, microwaves for cooking, infrared for heat detection, ultraviolet for sterilization, and visible light is what we can see. The wavelength of each region determines its energy and potential uses.
Examples & Analogies
Consider the different settings in a kitchen. Just as specific tools are designed for specific tasks (a microwave for heating, a blender for mixing), different parts of the electromagnetic spectrum suit different technological applications. For example, radio waves are perfect for sending music and news through the air to our radios, while infrared rays help us heat food quickly.
Measuring Electromagnetic Radiation
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Differently kinds of units are used to represent electromagnetic radiation. These radiations are characterised by the properties, namely, frequency (ν) and wavelength (λ). The SI unit for frequency (ν) is hertz (Hz, s–1), defined as the number of waves that pass a given point in one second. Wavelength should have the units of length, and the SI units of length is meter (m).
Detailed Explanation
Electromagnetic radiation is quantified using two primary characteristics: frequency and wavelength. Frequency indicates how many wave cycles occur in a second (measured in hertz), while wavelength measures the distance between successive peaks of a wave (measured in meters). This relationship is crucial for understanding how different types of electromagnetic radiation behave.
Examples & Analogies
Think of waves at the beach. The number of waves crashing on the shore in one minute is like the frequency, while the distance between each wave crest is akin to the wavelength. Understanding both helps surfers determine the best time to catch a wave and assess the ocean's behavior.
Speed of Light
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In vacuum all types of electromagnetic radiations, regardless of wavelength, travel at the same speed, i.e., 3.0 × 10^8 m s–1 (2.997925 × 10^8 m s–1, to be precise). This is called speed of light and is given the symbol ‘c’. The frequency (ν), wavelength (λ), and velocity of light (c) are related by the equation c = νλ.
Detailed Explanation
The speed of light in a vacuum is constant for all electromagnetic waves, which is a cornerstone of physics. This means that no matter the type of electromagnetic wave, they all travel at the same incredible speed. The relationship between speed, frequency, and wavelength is crucial; it indicates that as one value increases, the others adjust accordingly to keep the equation balanced.
Examples & Analogies
Imagine a highway where all vehicles (representing different types of electromagnetic waves) can travel at the same maximum speed. If larger vehicles (like slow-moving radio waves) take up more space (longer wavelength), then fewer can fit in front of them, while faster-moving vehicles (like X-rays) can travel shorter distances but reach their destinations quicker, demonstrating the set speed limit.
Key Concepts
-
Electromagnetic Waves: Represent the propagation of energy through oscillating electric and magnetic fields.
-
Wave-Particle Duality: States that light and other forms of electromagnetic radiation exhibit qualities of both waves and particles.
-
Quantization of Energy: The concept that energy is emitted or absorbed in discrete amounts.
-
Planck's Constant: A fundamental constant related to the quantization of energy, crucial in quantum mechanics.
Examples & Applications
The use of microwaves in cooking demonstrates the practical application of electromagnetic waves to heat food efficiently.
In medicine, X-rays provide valuable diagnostic images by utilizing high-frequency electromagnetic radiation.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Maxwell's waves, in blue and white, spread through the air, a beautiful sight!
Stories
Once upon a time, two friends, Wave and Particle, journeyed together. They encountered many phenomena and realized they could switch roles whenever they wished, captivating everyone they met.
Memory Tools
To remember the electromagnetic spectrum: 'Raging Martians Invaded Venusians Using X-ray Guns' (Radio, Microwave, Infrared, Visible, Ultraviolet, X-ray, Gamma).
Acronyms
Remember EMR for Electromagnetic Radiation.
Flash Cards
Glossary
- Electromagnetic Waves
Waves that are propagated by simultaneous oscillations of electric and magnetic fields.
- Dual Nature
The concept that electromagnetic radiation exhibits both wave-like and particle-like properties.
- Quantum Theory
A theory that describes how electromagnetic energy is emitted and absorbed in discrete packets called quanta.
- Photon
A quantum of light or electromagnetic radiation.
- Wavelength (λ)
The distance between successive crests of a wave, commonly measured in meters.
- Frequency (ν)
The number of waves that pass a given point in a specific amount of time, typically expressed in hertz (Hz).
- Planck's Constant (h)
A fundamental constant used to describe the sizes of quanta, equal to approximately 6.626 x 10–34 Js.
Reference links
Supplementary resources to enhance your learning experience.
