Charge on the Electron
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the Electron
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are diving into the fundamental particle that is essential to atomic structure: the electron. Can anyone tell me what they know about the electron?

Isn't it negatively charged?

Yes! The electron is indeed negatively charged. R.A. Millikan's experiments helped us quantify that charge. But first, does anyone know who discovered the electron?

J.J. Thomson discovered it in the early 1900s.

Exactly! Thomson's work used cathode rays to identify the electron. He showed that it has a negative charge and a very small mass. Now, can anyone explain why this discovery was important for chemistry?

It helps us understand atomic structure and chemical bonding.

Right! The electron's behavior fundamentally influences how atoms bond and interact. Let's dig deeper into Millikan's experiment which provides the precise value of this charge.
Millikan's Oil Drop Experiment
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Millikan's oil drop experiment is a pivotal moment in physics. Who can summarize how he performed this experiment?

He suspended tiny charged oil droplets in an electric field and measured their motion, right?

Correct! By balancing the electric and gravitational forces on these droplets, he could calculate the charge of a single electron. Why do you think this methodology was so effective?

Because he could isolate the effects of charge in a controlled environment.

Precisely! And what value did he determine for the charge of an electron?

About -1.602 × 10^-19 coulombs.

Well done! This value is now fundamental in our understanding of electric charge in matter. Let's remember this for our upcoming exercises.
Implications of Electron Charge
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we know the charge of the electron, let’s discuss its implications. How does the negative charge of electrons affect chemical reactions?

It means they can attract positively charged protons, which helps atoms bond.

Exactly! The interaction between electrons and protons underpins atomic stability and the formation of molecules. Can you infer what would happen if electrons did not have this charge?

Atoms wouldn't be able to form bonds, and there’d be no molecules.

Correct! Understanding the electron’s charge helps us predict chemical reactivity and the behavior of substances.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the charge of the electron is investigated through Millikan's oil drop experiment, revealing that the charge is approximately -1.602176 × 10^-19 C. This fundamental constant plays a vital role in the behavior of charged particles and the formation of atoms.
Detailed
Charge on the Electron
Overview
This section explores the concept of the charge on the electron, a critical aspect of atomic structure that influences the behavior of subatomic particles. The values for charge and mass were obtained primarily through the work of physicists J.J. Thomson and R.A. Millikan.
Millikan’s Oil Drop Experiment
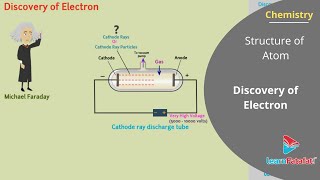
R.A. Millikan conducted a famous oil drop experiment between 1906 and 1914 to measure the charge of the electron. The experiment involved suspending charged oil droplets between two plates, allowing Millikan to precisely measure the forces acting on the drops. By balancing gravitational forces with electrical forces, he deduced the charge on an electron to be approximately -1.602176 × 10^-19 C. This groundbreaking study confirmed that the electron carries a fundamental charge, essential for understanding electric charge interactions in both atoms and molecules.
Implications
The determination of the electron's charge is crucial for various fields of physics and chemistry. It enhances our comprehension of atomic and electronic structures, facilitating the understanding of electron arrangements in atoms, which are foundational to chemical bonding and reactions.
In conclusion, the charge on the electron reinforces the principles of atomic theory, playing a significant role in current models of atomic behavior and interactions.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Millikan's Oil Drop Experiment
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
R.A. Millikan (1868-1953) devised a method known as oil drop experiment (1906-14) to determine the charge on the electrons. He found the charge on the electron to be –1.6 × 10–19 C. The present accepted value of electrical charge is –1.602176 × 10–19 C. The mass of the electron (me) was determined by combining these results with Thomson’s value of e/me ratio.
Detailed Explanation
Millikan conducted the oil drop experiment to measure the charge of an electron. By observing tiny charged oil droplets suspended in an electric field, he could calculate the charge by balancing the gravitational force on the droplets with the electrostatic force exerted on them. His findings showed that the charge of an electron is approximately -1.6 x 10^-19 coulombs. This experiment was crucial in establishing the quantized nature of electric charge.
Examples & Analogies
Imagine the oil droplets as tiny balloons in a windy room. If you want to keep them stationary, you can use a fan (the electric field). By adjusting the speed of the fan, you can find the right balance that stops the balloons from floating away. Similarly, Millikan’s experiment involved balancing forces to get an exact measurement of charge.
Charge Determination and Mass of Electron
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The charge on the electron was determined in Millikan's experiment. The calculated mass of the electron (me) using Thomson’s value of e/me ratio is 9.1094×10–31 kg.
Detailed Explanation
While Millikan focused on the measurement of charge, he also provided insights into the mass of the electron. By combining the results of his experiment with J.J. Thomson's earlier work on the charge-to-mass ratio of the electron, Millikan was able to quantify the mass. The accepted value indicates that electrons are very light, making them significant in atomic and molecular structures, despite their tiny size.
Examples & Analogies
Think of the electron as a very light feather compared to a heavy rock (which represents protons and neutrons). Even though the feather is much lighter than the rock, it still plays a vital role in how it interacts with the world around, just as electrons influence chemical reactions despite their small mass.
Key Concepts
-
Charge of the Electron: The charge is approximately -1.602176 × 10^-19 coulombs.
-
Millikan's Oil Drop Experiment: This pivotal experiment verified the quantized nature of electric charge.
-
Subatomic Particles: Electrons are one of the three main subatomic particles, influencing atomic structure and interactions.
Examples & Applications
Millikan's oil drop experiment demonstrated how an electron's charge can be deduced through analyzing the motion of charged droplets in an electric field.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Electrons are small, negatively neat, / Their charge, in coulombs, can’t be beat!
Stories
In a science lab, a brave physicist named Millikan dropped tiny oil droplets to uncover the secret charge of the electron.
Memory Tools
MCQ: Remember the charge on the 'Electron' is 'Minus One' followed by 602.
Acronyms
M.O.D.E.
Millikan's Oil Drop Experiment!
Flash Cards
Glossary
- Electron
A negatively charged subatomic particle found in atoms.
- Charge
An electrical property of particles that determines their electromagnetic interactions.
- Millikan's Oil Drop Experiment
An experiment conducted by R.A. Millikan that measured the charge of the electron.
Reference links
Supplementary resources to enhance your learning experience.
