Particle Nature of Electromagnetic Radiation: Planck’s Quantum Theory
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Black-body Radiation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning, class! Let's start by discussing black-body radiation. Can anyone tell me what a black body is?

Isn't it an object that absorbs all radiation?

Exactly! A black body absorbs all radiation and emits it uniformly. Now, why do you think this concept was important for Planck?

Because it helped explain why hot bodies emit different colors of light based on temperature?

Yes! As temperature increases, the color changes from red to blue. Can you remember the relationship between temperature and wavelength using the acronym T-B-R (Temperature - Blue - Red)?

That's a great way to remember it!

Fantastic! The intensity of radiation also changes with temperature. Let's summarize this concept.

In summary, a black body is a perfect absorber and emitter of radiation, and its properties change with temperature.
Planck's Quantum Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s dive into Planck’s quantum theory. Can someone summarize what Planck proposed regarding energy?

He proposed that energy is quantized, meaning it can only exist in discrete amounts.

Exactly! He introduced the idea that energy is emitted in quanta and coined the term 'quantum'. Why do you think this was a departure from classical physics?

Because classical physics thought energy was continuous?

Correct! This significant shift helped explain phenomena like black-body radiation. Let’s remember it with the mnemonic 'Q-Cube' for Quantum and Cubic Energy levels.

That makes it easier to remember!

Great! In summary, Planck’s theory reveals that energy levels are quantized, influencing how we understand radiation.
Photoelectric Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on to the photoelectric effect, how do we define this phenomenon?

It’s when light causes electrons to be emitted from a metal surface!

Exactly! In this effect, we noticed some surprising findings. Can anyone explain why the brightness of light affects the number of electrons emitted but not their kinetic energy?

Because energy depends on frequency, not brightness!

Well said! To remember this, think about 'Frequency is the Key' – where frequency determines the energy of the emitted electrons.

That's a unique way to recall it!

In summary, the photoelectric effect illustrates light’s dual nature: as both a wave and a particle, where frequency, not intensity, governs energy.
Dual Nature of Light
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s discuss the dual nature of light. Why is this concept crucial in understanding electromagnetic radiation?

Because it helps explain different behaviors like diffraction and interference?

Exactly! Light exhibits wave behavior through phenomena like diffraction, yet demonstrates particle behavior during interactions, like in the photoelectric effect. To remember this, use 'Waves Particles Dance'!

That’s catchy!

In summary, light has dual characteristics, acting as both wave and particle depending on the context.
Applications of Quantum Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Lastly, let’s examine how Planck's quantum theory has influenced modern physics. Why do we care about quantization today?

Because it’s the foundation for quantum mechanics and many technologies!

Exactly! Quantum theory underpins technology advancements such as semiconductors and lasers. Remember: 'Quantum Roots Tech' to connect quantum principles to tech applications.

That's a great way to connect it to real life!

In summary, understanding quantum concepts allows us to innovate and advance technologies crucial to our daily lives.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses how certain phenomena like black-body radiation and the photoelectric effect could not be explained by classical physics, leading to Max Planck's breakthrough in 1900. Planck's theory introduced the concept of quantization of energy, resulting in a better understanding of radiation emission, absorption, and the dual nature of light.
Detailed
Detailed Summary
This section delves into the particle nature of electromagnetic radiation, as articulated in Planck’s Quantum Theory. Classical physics struggled to explain several experimental observations, including the behavior of black-body radiation, the photoelectric effect, the variation in solid heat capacity with temperature, and the line spectra of atoms. Notably, Planck’s 1900 work highlighted that energy is quantized—meaning systems can only absorb or emit discrete energy amounts (quanta).
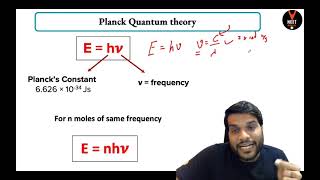
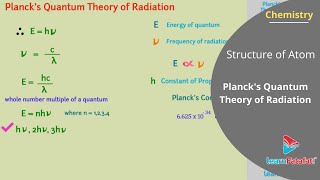
Planck introduced the concept of the black body, an idealized object that perfectly absorbs and emits radiation, with properties profoundly influenced by temperature. His famous equation, E = hν, denotes that the energy of a quantum is proportional to its frequency, where h is Planck’s constant.
The section further discusses the photoelectric effect, experimentally observed by Hertz, wherein electrons are emitted from a metal surface when exposed to light of sufficient frequency. This phenomenon contradicts classical physics expectations, as energy transfer is instant, and energy ejection depends on frequency, not intensity. Einstein later applied Planck’s theory to explain these observations, affirming the dual wave-particle nature of light, which remains crucial for understanding modern physics.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Quantum Theory and Black Body Radiation
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Some of the experimental phenomenon such as diffraction and interference can be explained by the wave nature of the electromagnetic radiation. However, following are some of the observations which could not be explained with the help of even the electromagnetic theory of 19th-century physics (known as classical physics):
(i) the nature of emission of radiation from hot bodies (black-body radiation)
(ii) ejection of electrons from metal surface when radiation strikes it (photoelectric effect)
(iii) variation of heat capacity of solids as a function of temperature.
Detailed Explanation
In this chunk, we mention that although the wave theory of electromagnetic radiation can explain phenomena like diffraction and interference, it fails to explain several critical observations. Specifically, black-body radiation refers to how hot objects emit electromagnetic radiation across different wavelengths, which classical physics struggled to interpret. Additionally, the photoelectric effect involves electrons being released from metals when exposed to light, which also could not be adequately described using classical theories.
Examples & Analogies
Think of a blacksmith’s forge where the metal heats up. As it gets hotter, it glows and changes color, emitting light. This is like the black-body radiation effect, but classical physics can't accurately predict how much energy and what wavelengths are emitted as the temperature changes.
Max Planck’s Contribution
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Max Planck arrived at a satisfactory relationship by making an assumption that absorption and emission of radiation arise from oscillators i.e., atoms in the wall of a black body. His theory suggested that radiation could be sub-divided into discrete chunks of energy, and he introduced the concept of 'quanta.' The energy (E) of a quantum of radiation is proportional to its frequency (ν) and is expressed by the equation (E = hν), where 'h' is Planck's constant (6.626×10–34 J·s).
Detailed Explanation
Planck theorized that energy could not be emitted or absorbed continuously, but rather in discrete amounts called 'quanta.' This means that instead of being able to have any energy level, atoms can only absorb or emit specific amounts of energy. The relationship between energy and frequency is given by the formula E = hν, which means that as the frequency of radiation increases, so does its energy. This was revolutionary and laid the groundwork for future quantum theories.
Examples & Analogies
Imagine walking up steps of a ladder. You can only stand on the steps (discrete energy levels) and cannot float in between. Similarly, electrons in atoms can only have certain energy levels and not any value in between, just like you can only be on a step or the ground, not in the space between them.
Photoelectric Effect Explained
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In 1887, H. Hertz performed a very interesting experiment in which electrons (or electric current) were ejected when certain metals (for example potassium, rubidium, caesium) were exposed to a beam of light. The results observed in this experiment include:
(i) The electrons are ejected from the metal surface as soon as the beam of light strikes the surface, with no time lag between the striking of the light beam and the ejection of electrons.
(ii) The number of electrons ejected is proportional to the intensity or brightness of light.
(iii) For each metal, there is a characteristic minimum frequency, ν0 (threshold frequency), below which photoelectric effect is not observed. At a frequency ν >ν0, the ejected electrons come out with certain kinetic energy.
Detailed Explanation
This chunk explains the photoelectric effect, which demonstrates how light interacts with matter. When light hits a metal surface, if its frequency is above a certain threshold, it can eject electrons from that metal. The key observations include that electrons are expelled almost immediately upon light exposure, the number of electrons correlates with the light's brightness, and a specific minimum frequency is required for this effect to occur. If the light frequency is below this threshold, no electrons are emitted, demonstrating that light behaves like particles (photons).
Examples & Analogies
Think about a trampoline where you need to bounce high enough to get over the edge. The trampoline represents the metal surface, and the energy of the jump (like the light's frequency) impacts whether or not you can make it over the edge (eject an electron). If your jump isn't powerful enough (below threshold frequency), you bounce back down without going over.
Dual Behavior of Light
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The particle nature of light posed a dilemma for scientists. On the one hand, it could explain the black body radiation and photoelectric effect satisfactorily but on the other hand, it was not consistent with the known wave behavior of light which could account for the phenomena of interference and diffraction.
Detailed Explanation
In this chunk, we summarize the conflict between light's wave and particle nature. Scientists found that light behaves both as a wave (exhibiting properties like interference and diffraction) and as a particle (explaining phenomena like the photoelectric effect). This dual nature was a key realization in understanding quantum mechanics and suggested a deeper, more complex relationship between light and energy.
Examples & Analogies
You can think of how a foghorn functions—it produces sound waves (wave behavior), but can also be thought of as individual sound 'packets' (particles). Just as sound can behave in both ways, so can light. This dual nature can be tricky to understand initially, but like fog horns, both waves and particles have their own roles and moments when they stand out.
Key Concepts
-
Black Body: An idealized object that completely absorbs and emits radiation.
-
Quantization: Energy can only exist in discrete amounts called quanta.
-
Photoelectric Effect: Emission of electrons from a material when exposed to light of certain frequencies.
-
Planck’s Constant: A key constant in quantum physics that relates energy and frequency.
-
Threshold Frequency: The minimum frequency necessary for the photoelectric effect.
-
Wave-Particle Duality: The concept that light exhibits both wave-like and particle-like properties.
Examples & Applications
When heating an iron rod, it first emits red light, then white, and finally blue light as temperature increases, demonstrating black-body radiation.
In the photoelectric effect experiment, light of a certain frequency hits potassium metal, and only light with a frequency above the threshold can eject electrons.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Planck's light so bright, ejected electrons take flight, quantized by night, wave and particle in sight.
Stories
Imagine a tiny staircase where each step represents an energy quantum; electrons jump from one step to the next when hit by light, never in between.
Memory Tools
Use 'F-QE' to remember that Frequency Quantifies Electrons in the photoelectric effect.
Acronyms
Remember 'B-P-Q' for Black body, Planck’s constant, and Quantum energy!
Flash Cards
Glossary
- Black body
An idealized physical object that absorbs all incoming radiation and re-emits it perfectly at all frequencies.
- Quantum
The smallest discrete quantity of energy that can be emitted or absorbed, as defined by Planck.
- Photoelectric Effect
The phenomenon where electrons are ejected from a metal surface when exposed to light of sufficient frequency.
- Planck’s Constant (h)
The proportionality constant (6.626 × 10^−34 J s) that relates the energy of a photon to its frequency.
- Threshold Frequency (ν0)
The minimum frequency of light required to eject an electron from a material.
- Waveparticle duality
The concept that light and matter exhibit both wave-like and particle-like properties.
Reference links
Supplementary resources to enhance your learning experience.
