Quantum Mechanical Model of Atom
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Quantum Mechanics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we delve into the Quantum Mechanical Model of the atom. Let's begin by discussing why previous models, like those of Dalton and Bohr, could not explain many atomic phenomena.

Were those models completely wrong?

Not wrong, but limited. While Bohr's model worked for hydrogen, it couldn't explain multi-electron atoms. What do we need for a more comprehensive model?

Maybe we need to consider wave behavior instead of just particles?

Exactly! This leads us to the introduction of Schrödinger’s wave equation, which we will explore next.
Schrödinger's Wave Equation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Schrödinger's equation describes how the quantum state of a physical system changes over time. Can anyone tell me what a wave function represents?

Is it a probability distribution for where we might find an electron?

Correct! The wave function gives us the probability density |ψ|², which helps us identify where an electron is likely to be.

So, electrons aren’t just in fixed paths anymore?

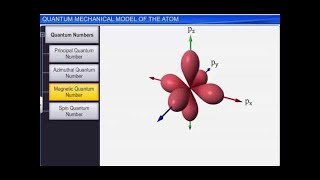
Exactly! They exist in orbitals defined by quantum numbers. Now, who can list these quantum numbers?

There’s the principal quantum number, azimuthal quantum number, and magnetic quantum number.

Right! Each number helps define various aspects of an electron's position and energy.
Understanding Quantum Numbers
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s go deeper into quantum numbers. The principal quantum number 'n' describes the energy level, and 'l' defines the subshell shape. Does anyone remember the shapes these define?

Yes! 's' orbitals are spherical, 'p' orbitals are dumbbell-shaped, and 'd' orbitals have more complex shapes.

Excellent! And what about 'ml', the magnetic quantum number?

It describes the orientation of the orbital in space, right?

Yes! Each set of orbitals has designated orientations that affect chemical bonding.
Uncertainty Principle and Probability
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The Heisenberg Uncertainty Principle states that you cannot precisely know both the position and momentum of an electron. How does this change our understanding of atomic structure?

It means we can only discuss probabilities, rather than exact paths.

Exactly correct! Because of this, we talk about where an electron is most likely to be found, rather than where it is.
Applications of the Quantum Mechanical Model
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s consider how the quantum model applies to multi-electron atoms. Can someone explain how their orbitals are filled?

They follow the Aufbau principle and fill from lower to higher energy orbitals.

Right! And what about the Pauli Exclusion Principle?

It states that no two electrons can have the same set of quantum numbers.

Correct! This reinforces the idea of stability in electronic configurations. Now, as a summary, would someone like to highlight what we learned today?

We discussed quantum mechanics, the Schrödinger equation, quantum numbers, the uncertainty principle, and how these shape atomic behavior.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores the development of the quantum mechanical model of the atom, highlighting the need for this model due to the limitations of previous models. It introduces important concepts, such as the Schrödinger equation, the dual nature of electrons, and how quantum numbers define electron behavior and energy levels in atoms.
Detailed
Quantum Mechanical Model of Atom
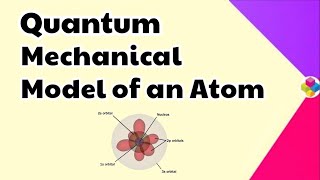
The quantum mechanical model emerges from earlier atomic theories that struggled to accurately describe atomic behavior. Unlike the Bohr model that depicts electrons in fixed orbits, the quantum mechanical model utilizes wave functions to describe the probability of finding electrons in various regions around the nucleus. This shift recognizes the dual nature of electromagnetic radiation and matter, encapsulated in the Schrödinger equation, which serves as the foundation for modern quantum mechanics. The model emphasizes that electrons reside in atomic orbitals characterized by quantum numbers, enhancing our understanding of electronic configurations, chemical properties, and spectroscopic phenomena.
Key Concepts and Features
- Energy Quantization: Electrons can only occupy certain energy levels, leading to discrete energy states.
- Wave Function: The solution to the Schrödinger equation provides information about the likelihood of finding an electron in a particular region of space.
- Uncertainty Principle: There's a fundamental limit to simultaneously knowing an electron's exact position and momentum.
- Quantum Numbers: Electrons are defined by a set of quantum numbers that describe their energy, shape, and orientation within an atom.
The applications of quantum mechanics extend beyond the hydrogen atom, influencing our comprehension of multi-electron systems and the chemical behavior of elements.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Quantum Mechanics
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Classical mechanics, based on Newton’s laws of motion, successfully describes the motion of all macroscopic objects such as a falling stone, orbiting planets etc., which have essentially a particle-like behaviour as shown in the previous section. However, it fails when applied to microscopic objects like electrons, atoms, molecules etc. This is mainly because of the fact that classical mechanics ignores the concept of dual behaviour of matter especially for sub-atomic particles and the uncertainty principle.
Detailed Explanation
Quantum mechanics emerged as a new branch of physics to address the limitations of classical mechanics. While classical mechanics accurately explains large-scale motions, it struggles with small-scale phenomena such as atoms and subatomic particles. This failure is due to classical mechanics not accounting for the wave-particle duality of particles and the uncertainties involved in measuring their properties.
Examples & Analogies
Consider how we can accurately predict the path of a car on a road (classical mechanics), but when we try to predict where a tiny particle like an electron will be, our calculations become probabilistic rather than certain. It's similar to predicting the exact position of a firefly in a dark room – we can only estimate where it might be based on its behavior.
Schrödinger Equation
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Quantum mechanics was developed independently in 1926 by Werner Heisenberg and Erwin Schrödinger. Here, however, we shall be discussing the quantum mechanics which is based on the ideas of wave motion. The fundamental equation of quantum mechanics was developed by Schrödinger and it won him the Nobel Prize in Physics in 1933. This equation which incorporates wave-particle duality of matter as proposed by de Broglie is quite complex and knowledge of higher mathematics is needed to solve it.
Detailed Explanation
Schrödinger's equation describes how the quantum state of a physical system changes over time. It is central to quantum mechanics, providing a way to compute the probabilities of finding a particle in a particular position and state. The solutions to this equation give rise to wave functions which describe the behavior of quantum particles.
Examples & Analogies
Think of Schrödinger's equation like a recipe for baking a cake. Just as the right ingredients and instructions yield a delicious cake, the Schrödinger equation combines the fundamental aspects of quantum mechanics to produce a complete description of a particle’s behavior.
Energy Levels and Quantum Numbers
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The solutions of Schrödinger's equation for the hydrogen atom give quantized energy levels shown by the principal quantum number n, azimuthal quantum number l, and magnetic quantum number ml. The energy of electrons in atoms is quantized (i.e., can only have certain specific values).
Detailed Explanation
In the quantum mechanical model, electrons occupy specific energy levels, and they cannot exist in between these levels. Each level is defined by quantum numbers, which determine the size, shape, and orientation of the orbital that the electron occupies. The quantization of energy means that electrons can only exist at certain levels, resulting in distinct energy states.
Examples & Analogies
Imagine a staircase: just like you can only stand on the steps and not in the air between them, electrons can only exist in specific energy levels and not anywhere in between. Each step corresponds to a quantized energy level.
The Concept of Atomic Orbitals
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An atomic orbital is the wave function ψ for an electron in an atom. Whenever an electron is described by a wave function, we say that the electron occupies that orbital. The probability of finding an electron at a point within an atom is proportional to the |ψ|² at that point.
Detailed Explanation
Atomic orbitals represent the regions in an atom where there is a high probability of finding an electron. The wave function provides a mathematical description of the electron, while the square of this wave function gives the probability density, indicating how likely it is to find the electron in various locations around the nucleus.
Examples & Analogies
Think of an atomic orbital as a cloud around a nucleus. The density of this cloud at any point indicates how likely you are to find an electron there, similar to how a dense fog is likely to have more water droplets in some areas than in others.
Importance of Quantum Mechanical Model
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The quantum mechanical model of the hydrogen atom successfully predicts all aspects of the hydrogen atom spectrum including some phenomena that could not be explained by the Bohr model.
Detailed Explanation
The quantum mechanical model has proven to be more accurate than previous atomic models, including Bohr's, particularly because it accounts for the wave nature of electrons. This model allows scientists to understand and predict the behavior of electrons in various atoms, which is foundational for understanding chemical bonding and reactions.
Examples & Analogies
Just as modern navigation systems use GPS satellites to provide accurate locations, quantum mechanics uses wave functions to accurately predict the positions and behaviors of subatomic particles. The advancements in technology stem from the reliable predictions made by the quantum mechanical model.
Key Concepts
-
Energy Quantization: Electrons can only occupy certain energy levels, leading to discrete energy states.
-
Wave Function: The solution to the Schrödinger equation provides information about the likelihood of finding an electron in a particular region of space.
-
Uncertainty Principle: There's a fundamental limit to simultaneously knowing an electron's exact position and momentum.
-
Quantum Numbers: Electrons are defined by a set of quantum numbers that describe their energy, shape, and orientation within an atom.
-
The applications of quantum mechanics extend beyond the hydrogen atom, influencing our comprehension of multi-electron systems and the chemical behavior of elements.
Examples & Applications
An electron in a hydrogen atom can exist in several quantized energy states, predicted by the Schrödinger equation.
The distribution of electron cloud in atomic orbitals can help predict chemical bonding and reactivity of elements.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the atom’s dance, wave and particle prance, uncertainty reigns in quantum finance!
Stories
Once, in a world of tiny terms, electrons swirled in quantum spirals; not in fixed paths but in fuzzy clouds of chance, exploring their fate both near and far.
Memory Tools
Remember 'PAMU' for quantum numbers: 'P' for principal, 'A' for azimuthal, 'M' for magnetic, and 'U' for up (spin).
Acronyms
Use 'QUMP' to remember
'Q' for Quantum
'U' for Uncertainty
'M' for Model
'P' for Particles.
Flash Cards
Glossary
- Quantum Mechanics
A branch of physics that deals with the behavior of subatomic particles and incorporates the wave-particle duality.
- Wave Function (ψ)
A mathematical function that describes the probability distribution of an electron's position in an atom.
- Principal Quantum Number (n)
Indicates the energy level and size of an orbital.
- Azimuthal Quantum Number (l)
Describes the shape of an orbital.
- Magnetic Quantum Number (ml)
Indicates the orientation of an orbital in space.
- Heisenberg Uncertainty Principle
States that the position and momentum of a particle cannot both be precisely determined at the same time.
Reference links
Supplementary resources to enhance your learning experience.
