Heisenberg’s Uncertainty Principle
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Uncertainty Principle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are exploring the Heisenberg Uncertainty Principle. This principle fundamentally changes how we view particles at the quantum level. What do you think it means when we say something is 'uncertain'?

I think it means we can't measure something precisely.

Exactly! The principle states that the more accurately we know an electron's position, the less accurately we can know its momentum, and vice versa. This is mathematically represented as Δx · Δp ≥ ℏ / 2. Can anyone tell me what ℏ represents?

Isn't ℏ the reduced Planck's constant?

Great job! It shows how small scales work differently than our everyday experiences. The principle introduces fuzziness in measurements, unlike definite positions we expect in classical physics. Remember, this is crucial in quantum mechanics where particles behave very differently.
Implications of Uncertainty
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive deeper into the implications of this principle. How do you think the Uncertainty Principle affects our understanding of electrons in an atom?

Maybe it means we can't know exactly where they are?

Correct! It suggests that electrons do not have definite paths; rather, we can only speak of probabilities of finding them in certain regions. This uncertainty is essential in developing atomic models. Can someone provide an example of how this might affect a chemical reaction?

If we can't pinpoint an electron's location, can we predict how molecules will react?

Precisely! It makes predicting reactions more complex, as electrons can be spread out over areas rather than at fixed points, affecting bonding and reactions.
Classical vs Quantum Physics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's compare classical physics to quantum mechanics in light of the Uncertainty Principle. What is the main difference?

Classical physics assumes we can measure everything accurately, while quantum mechanics says we can't.

Exactly! In classical mechanics, we can observe and predict motion precisely. However, quantum mechanics acknowledges limitations and inaccuracies in our observations, reshaping our understanding of atomic behavior.

So, does that mean in quantum physics, we often deal with probabilities instead of certainties?

Yes! Probabilities play a key role in quantum mechanics, informing us about the likelihood of finding particles in certain states or locations.
Measurement Challenges
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How does measuring something like the position of an electron affect our results?

If we measure its position really accurately, we lose details about its momentum?

Exactly! That's why in quantum experiments, measuring tools must be incredibly precise, or they can significantly alter the state of what's being observed, illustrating the principle's limitations.

So we can never fully know everything about a particle at once?

Correct! This inherent uncertainty is what distinguishes quantum events from the predictable nature of classical mechanics. It opens new avenues for research and technological innovation, particularly in the fields of quantum computing and advanced electronics.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Heisenberg's Uncertainty Principle, articulated in 1927, emphasizes the inherent limitations in measuring pairs of physical properties, such as position and momentum, at the quantum level. This principle signifies the transition from classical to quantum mechanics, challenging previous notions of definiteness in particle behavior.
Detailed
Heisenberg’s Uncertainty Principle
The Heisenberg Uncertainty Principle is a fundamental concept in quantum mechanics proposed by Werner Heisenberg in 1927. It states that it is impossible to determine simultaneously both the exact position (
∆x) of a particle, such as an electron, and its exact momentum (
∆p) (or velocity). Mathematically, this principle is represented as:
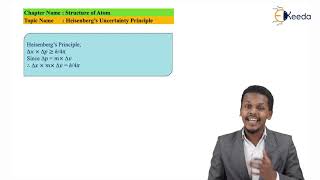
Δx · Δp ≥ ℏ / 2
Where ℏ (h-bar) is the reduced Planck's constant.
Key Implications
- Complementarity: The principle illustrates the dual wave-particle nature of matter. As one property (position) is measured more accurately, the uncertainty in the other property (momentum) increases.
- Quantum Behavior: It challenges classical physics' predictability, introducing the concept of 'fuzzy' measurements at the microscopic level and indicating that electrons do not have defined paths.
- Measurement Limitations: The act of measuring one property affects the other, echoing the concept that quantum systems are inherently probabilistic rather than deterministic.
The uncertainty principle has profound implications for studying atomic and subatomic particles, influencing the development of quantum mechanics and our understanding of the structure and behavior of matter.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to the Uncertainty Principle
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Werner Heisenberg a German physicist in 1927, stated uncertainty principle which is the consequence of dual behaviour of matter and radiation. It states that it is impossible to determine simultaneously, the exact position and exact momentum (or velocity) of an electron.
Detailed Explanation
The Heisenberg Uncertainty Principle articulates a fundamental limit to the accuracy with which certain pairs of physical properties of a particle, known as complementary variables or canonical variables, can be known. This principle signifies that the more accurately we know an electron's position (where it is), the less accurately we can know its momentum (how fast it's moving and in what direction). For example, if you know that an electron is precisely at a specific point in space, you cannot know how fast it is moving, and vice versa.
Examples & Analogies
Think of trying to catch a fast-moving butterfly. If you use a very slow and careful approach to ensure you're directly above it (position), it can easily fly away before you can react to catch it (momentum). Conversely, if you quickly approach where it has been (focusing on momentum), you might not be able to pinpoint exactly where it is in that moment.
Mathematical Representation
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Mathematically, it can be given as in equation (2.23).
Detailed Explanation
In its mathematical form, the uncertainty principle can be expressed as Δx * Δp ≥ h/4π, where Δx is the uncertainty in position, Δp is the uncertainty in momentum, and h is the Planck constant. This equation quantitatively expresses the trade-off between the precision of the position and momentum of a particle – the more you know about one, the less you know about the other. The constant h is a very small number, which makes the effect significant only at microscopic scales.
Examples & Analogies
Imagine trying to estimate the height of a building while standing at a distance. If you move closer to the building, you can accurately gauge its height (precision in position), but you lose sight of the entire scope around it (momentum). This translates directly to the principle – definitive knowledge of one aspect precludes the other.
Implications of the Uncertainty Principle
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If the position of the electron is known with high degree of accuracy (Δx is small), then the velocity of the electron will be uncertain [Δ(vx) is large]. On the other hand, if the velocity of the electron is known precisely (Δ(vx) is small), then the position of the electron will be uncertain (Δx will be large).
Detailed Explanation
This aspect of the uncertainty principle emphasizes the inherent limitations of measurement at quantum levels. When scientists try to pinpoint the location of an electron with great precision, the calculations about its speed become less reliable – the electron’s movement becomes more unpredictable. Hence, in quantum mechanics, we often talk about probabilities rather than certainties. Thus, we describe where the electron might be rather than where it definitively is.
Examples & Analogies
Consider a person trying to track a quick-moving soccer ball. If they focus intently on the ball's precise location, they might fail to notice how fast the ball is moving and where it might roll next. But if they instead try to gauge its speed, they could lose track of where exactly the ball is on the field.
Understanding the Measurement Problem
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To observe an electron, we can illuminate it with “light” or electromagnetic radiation. The “light” used must have a wavelength smaller than the dimensions of an electron.
Detailed Explanation
When measuring small particles like electrons, scientists use light such as lasers to 'see' the particle. However, to gain accurate information about an electron's position, the wavelength of light used must be much smaller than the size of the electron itself. This presents a problem as the very act of measuring affects the electron – shining light on it alters its position and momentum, again illustrating the uncertainty principle in practice.
Examples & Analogies
This scenario can be compared to trying to observe a tiny ant using a magnifying glass. If you use a high-power lens, you can clearly see the ant, but getting too close changes its direction or even scares it away. The light (or observation) affecting the subject is akin to scientific measurement impacting electron behavior.
Key Concepts
-
Uncertainty Principle: A principle stating that position and momentum cannot be precisely known simultaneously.
-
Quantum Behavior: A behavior observed in particles at the quantum level, indicating non-predictable paths.
-
Wave-Particle Duality: The concept that particles, such as electrons, exhibit both wave-like and particle-like properties.
Examples & Applications
An electron's position can be measured to a high degree of accuracy, but this results in a significant uncertainty in velocity, according to Heisenberg's principle.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To know your spot and speed so bright, Heisenberg brings a fuzzy light.
Stories
Once there was a curious electron, always zipping about. It wanted to find its way home but Heisenberg told it, 'You can know where you are or go fast, but not both at once!'
Memory Tools
UP = Uncertainty Principle; Remember: Precise positions lead to fuzzy velocities.
Acronyms
PMP = Position, Momentum, Precision - reminding us of what cannot be precisely measured together.
Flash Cards
Glossary
- Heisenberg Uncertainty Principle
A fundamental theory in quantum mechanics stating that the position and momentum of a particle cannot both be precisely determined at the same time.
- Quantum Mechanics
The branch of physics that deals with the behavior of matter and light on the scale of atoms and subatomic particles.
- Planck's Constant
A fundamental constant that relates the energy of photons to their frequency, symbolized as h.
Reference links
Supplementary resources to enhance your learning experience.
