Stability of Completely Filled and Half Filled Subshells
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Ground State Electronic Configuration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’ll discuss how the ground state electronic configuration plays a role in the stability of an atom. Can someone explain what we mean by 'ground state'?

The ground state is the lowest energy state of an atom.

Exactly! And this state is crucial for an atom's stability. Now, when we refer to 'stability,' how might electronic configurations influence that?

If the configuration is lower in energy, shouldn't the atom be more stable?

Correct! Atoms with completely filled or half-filled subshells are generally more stable. Can anyone recall examples of such elements?

Copper and chromium are examples!

Great job! Let’s remember them as Cu and Cr. To recap, completely filled and half-filled configurations increase stability due to symmetrical distribution of electrons.
Symmetrical Electron Distribution
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s dive deeper into why symmetrical electron distribution leads to stability. Does anyone know how symmetry affects the forces between electrons and the nucleus?

Symmetry minimizes electron-electron repulsion, right?

Spot on! Less repulsion allows electrons to be held closer to the nucleus. Why is this important for an atom?

Because it means the electrons are more strongly attracted to the nucleus, contributing to stability.

Excellent! So when electrons are symmetrically arranged, the atom becomes less likely to react with others. The arrangement can also influence exchange energy. Can anyone explain what that means?

Uh, I think it’s about the energy released when electrons with the same spin exchange positions?

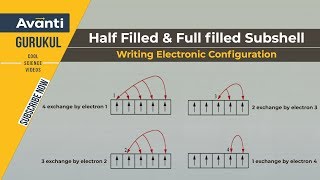
Exactly! This maximization of exchange energy occurs best in half-filled and completely filled orbitals, providing further stability.
Exchange Energy and Stability
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s talk about exchange energy in more detail. Why do you think it's significant in determining the stability of an atom?

It’s the extra stability that comes from having electrons with the same spins in orbitals, right?

Yes! So when electrons are arranged in a way that maximizes this exchange energy, what happens to the configuration?

The configuration becomes extra stable, like in half-filled or fully filled subshells!

Exactly! This explains why, in certain elements like chromium and copper, electrons shift from one subshell to another even if it means moving to a higher energy level. Let’s summarize what we've covered today.

We discussed three main points: The ground state configurations lead to stability, symmetrical distribution minimizes repulsions, and enhancement of stability through exchange energy.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Atoms exhibit enhanced stability when subshells are completely filled or half-filled due to their symmetrical electron distribution and the consequent increase in exchange energy, which contributes to the atom's overall stability.
Detailed
Stability of Completely Filled and Half Filled Subshells
The ground state electronic configuration of an element corresponds to the state with the lowest total electronic energy. While most atoms follow the basic rules of electron configuration, exceptions arise in elements like copper (Cu) and chromium (Cr) where electrons transition between subshells (4s and 3d) to achieve configurations that result in stability. This stability is associated with two main reasons: symmetrical electron distribution and maximum exchange energy.
Key Points:
- Symmetrical Distribution: Completely filled or half-filled subshells exhibit symmetry that leads to enhanced stability due to minimized electron shielding and stronger nuclear attraction.
- Exchange Energy: Additional stability arises when electrons with the same spin occupy degenerate orbitals, maximizing exchanges which release energy, contributing to stability.
Hence, configurations such as 3d^5 4s^1 for chromium and 3d^10 4s^1 for copper illustrate how access to additional stability can compel an electron shift from a lower energy subshell to one that is higher, provided that the resultant configuration is completely filled or half-filled.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Ground State Energy Configuration
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ground state electronic configuration of the atom of an element always corresponds to the state of the lowest total electronic energy.
Detailed Explanation
In chemistry, the electronic configuration of an atom is basically how its electrons are arranged in orbitals. The ground state refers to the arrangement where the electrons occupy the lowest available energy levels. This implies that atoms are stable when their electrons are in these configurations, minimizing their energy. For instance, if we look at an atom like calcium, its electrons fill up the 1s, 2s, 2p, 3s, 3p, and then move to the 4s orbital, as these are the lowest energy levels available.
Examples & Analogies
Consider a child stacking blocks: they will start by placing the smallest blocks at the bottom and then build up with larger blocks. Similarly, electrons fill the lowest energy levels before occupying higher ones, like stacking blocks efficiently, securing stability.
Electron Shift for Stability
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The electronic configurations of most of the atoms follow the basic rules given in Section 2.6.5. However, in certain elements such as Cu, or Cr, where the two subshells (4s and 3d) differ slightly in their energies, an electron shifts from a subshell of lower energy (4s) to a subshell of higher energy (3d), provided such a shift results in all orbitals of the subshell of higher energy getting either completely filled or half filled.
Detailed Explanation
In specific cases, like with copper (Cu) and chromium (Cr), the stability of the atom can be increased if an electron moves from a lower energy subshell (4s) to a higher energy subshell (3d). For chromium, the configuration becomes 3d5 4s1, and for copper, it is 3d10 4s1, instead of what one might expect (3d4 4s2 for Cr and 3d9 4s2 for Cu). This electron movement leads to a more stable, energetically favorable configuration due to the symmetrical distribution of electrons.
Examples & Analogies
Imagine a group of friends sitting in a room. If they have the option to rearrange their seating to make the atmosphere more balanced and enjoyable, they would do so. Just like friends seeking comfort, electrons rearrange to lower their energy and create a stable environment.
Reasons for Extra Stability
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It has been found that there is extra stability associated with these electronic configurations. The completely filled and completely half-filled subshells are stable due to the following reasons:
1. Symmetrical distribution of electrons: It is well known that symmetry leads to stability. The completely filled or half-filled subshells have a symmetrical distribution of electrons in them and are therefore more stable. Electrons in the same subshell (here 3d) have equal energy but different spatial distribution. Consequently, their shielding of one another is relatively small and the electrons are more strongly attracted by the nucleus.
2. Exchange Energy: The stabilizing effect arises whenever two or more electrons with the same spin are present in the degenerate orbitals of a subshell. These electrons tend to exchange their positions and the energy released due to this exchange is called exchange energy. The number of exchanges that can take place is maximum when the subshell is either half filled or completely filled.
Detailed Explanation
Filled subshells (like 3d10) and half-filled subshells (like 3d5) provide extra stability because of their symmetrical electron arrangement and minimal mutual shielding between electrons. This means that the electrons can interact less with each other and are held more tightly by the nucleus. Exchange energy also plays a crucial role. When electrons within a subshell have the same spin, the energy they release when they swap positions contributes to their stability. Thus, the arrangement of electrons in these configurations leads to a lower energy state overall, which is desirable.
Examples & Analogies
Think of a perfectly balanced seesaw. When two people of equal weight sit equidistant from the center, the seesaw is stable. This stability reflects how electrons are organized in completely filled or half-filled subshells. Their arrangement allows for minimal interaction (fluctuations), leading to a stable atomic structure, just like the well-balanced seesaw.
Key Concepts
-
Symmetrical distribution leads to increased stability.
-
Completely filled and half-filled subshells are energetically favorable.
-
Exchange energy maximization contributes to stability.
Examples & Applications
In chromium (Cr), the electron configuration is adjusted to 3d^5 4s^1 for stability.
In copper (Cu), the stable configuration is 3d^10 4s^1 instead of the expected 3d^9 4s^2.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Filled or half-filled, oh what a thrill, Stability grows when electrons chill!
Stories
Once in Atomland, electrons danced in shells, the completely filled ones rang joy bells!
Memory Tools
SHE - Shells, Half-filled, Energy - for remembering conditions of stability.
Acronyms
CHEERS
Complete and Half-filled Electrically Reduces Stability.
Flash Cards
Glossary
- Ground State
The lowest energy state of an atom, where it is most stable.
- Completely Filled Subshell
An electron subshell where all possible orbitals are occupied.
- HalfFilled Subshell
An electron subshell where each orbital contains one electron before pairing begins.
- Exchange Energy
The energy released when two electrons of the same spin exchange places in degenerate orbitals.
Reference links
Supplementary resources to enhance your learning experience.
