Electronic Configuration of Atoms
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Basics of Electronic Configuration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss electronic configuration. Can anyone tell me what they think electronic configuration means?

Is it how the electrons are arranged in an atom?

Exactly! It refers to the distribution of electrons in an atom's orbitals. This configuration is vital for determining how an atom behaves chemically.

Why is the arrangement of electrons so important?

Great question! The arrangement helps predict an element's reactivity, bonding characteristics, and properties. For example, noble gases have a complete outer shell, making them stable and non-reactive.

How do we know the order in which electrons fill the orbitals?

We follow the Aufbau principle, which states that electrons fill orbitals from the lowest to the highest energy level.

Can you give an example of this filling order?

Sure! The sequence goes from 1s to 2s, then to 2p, and so on. This order helps us determine configurations for different elements.

In summary, electronic configuration helps in understanding chemical behavior, and we follow specific principles to determine how electrons fill orbitals.
Principles Guiding Electronic Configuration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've grasped the basics, let’s dig deeper into the principles guiding electronic configuration. Who can recall the Pauli Exclusion Principle?

It states no two electrons can have the same set of quantum numbers, right?

That's correct! This principle implies that an orbital can hold a maximum of two electrons, but they must have opposite spins.

And what about Hund's rule?

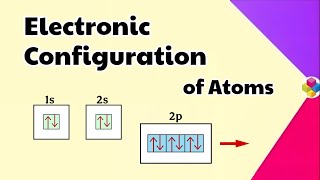
Good catch! Hund's rule states that electrons will fill degenerate orbitals singly before any pairing occurs. This maximizes stability.

How does this affect the electron configuration for something like phosphorus?

Great example! Phosphorus has an electronic configuration of 1s² 2s² 2p⁶ 3s² 3p³. You can see that in the 3p subshell, each orbital gets one electron first before pairing.

So, is the order of filling based solely on these principles?

Yes! Along with the Aufbau principle, they dictate how electrons arrange in an atom. Remember, understanding these rules is key to mastering chemistry!

In summary, Pauli's Exclusion Principle and Hund's Rule are essential for predicting electron configurations correctly.
Writing Electronic Configurations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s practice writing electronic configurations. Can someone tell me the configuration for helium?

Is it 1s²?

Yes, that's correct! Helium has two electrons occupying the 1s orbital. What about lithium?

It's 1s² 2s¹.

Exactly! Now, let's consider carbon.

1s² 2s² 2p²!

Perfect! Remember that the p orbitals fill after the s orbitals. And what would be the configuration for sodium?

1s² 2s² 2p⁶ 3s¹!

Great job! What about using the noble gas notation to simplify configurations?

We could write sodium as [Ne] 3s¹!

Exactly! This notation makes it easier to express electronic configurations, especially for larger elements. Let’s summarize what we learned today.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The electronic configuration of an atom describes how its electrons are distributed among various orbitals. The configurations are influenced by principles such as the Aufbau principle, Pauli exclusion principle, and Hund's rule, which dictate the order and manner in which electrons fill available orbitals.
Detailed
Electronic Configuration of Atoms
The electronic configuration of an atom refers to the distribution of its electrons in atomic orbitals. It is fundamental to understanding the chemical behavior of elements, including their reactivity and bonding properties.
Key Principles of Electronic Configuration
- Aufbau Principle: Electrons fill atomic orbitals starting from the lowest energy level to the highest. The typical order follows the pattern: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, etc.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of quantum numbers. This restricts each orbital to hold a maximum of two electrons with opposite spins.
- Hund's Rule of Maximum Multiplicity: When electrons occupy degenerate orbitals (orbitals of the same energy), one electron enters each orbital until all are half-filled before pairing begins.
Electronic Configuration Notation
Electronic configurations can be represented in two ways:
- Superscript Notation: Example: 1s² 2s² 2p⁶ for Neon.
- Orbital Diagram: This uses boxes or lines to represent orbitals and arrows pointing up and down to represent electrons.
Importance of Electronic Configuration
Understanding the electronic configuration helps in predicting:
- The chemical reactivity of elements
- The formation of bonds and molecular structures
- Trends in the periodic table, such as atomic size, ionization energy, and electronegativity.
Youtube Videos


![Structure Of Atom JEE L9 [Electronic Configuration] | Class 11 Chemistry | JEE 2023 | Nurture](https://img.youtube.com/vi/akEV2ZgM2C0/mqdefault.jpg)

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Electronic Configuration
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The distribution of electrons into orbitals of an atom is called its electronic configuration.
Detailed Explanation
Electronic configuration refers to how electrons are arranged in an atom's orbitals. The arrangement is significant because it determines the chemical properties of the element and how it interacts with other atoms.
Examples & Analogies
Think of electronic configuration like the seating arrangement in a classroom. Just as students fill seats in a way that optimizes their learning environment, electrons fill orbitals in an order that minimizes energy and stabilizes the atom.
Notations for Electronic Configuration
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The electronic configurations of different atoms can be represented in two ways. For example: (i) sapbdc ...... notation (ii) Orbital diagram.
Detailed Explanation
Two common notations for representing electronic configuration include the shorthand notation, which uses the letters s, p, d, and f along with superscripts that indicate the number of electrons, and the orbital diagram, where each orbital is represented by a box, and the electrons are indicated by arrows denoting their spins.
Examples & Analogies
Imagine you are organizing a party and have two ways to show where everyone sits: a guest list (the notation) and a seating chart (the orbital diagram). Each has its advantages, but together they give a clear picture of the arrangement.
Examples of Electron Configurations
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The hydrogen atom has only one electron which goes in the orbital with the lowest energy, namely 1s. The electronic configuration of the hydrogen atom is 1s1.
Detailed Explanation
For hydrogen, which has one electron, the configuration is very simple. The electron occupies the lowest energy orbital, which is the 1s orbital. As more electrons are added, their configurations become more complex, filling higher energy levels.
Examples & Analogies
Think of placing books in a shelf. The 1s orbital is like the bottom shelf where you place your heaviest and most frequently used books first. The next shelves (higher orbitals) get filled with lighter or less frequently used books as is needed.
Filling Pattern of Electrons
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The electronic configuration of the elements sodium (Na, 1s22s22p63s1) to argon (Ar, 1s22s22p63s23p6) follow exactly the same pattern as the elements from lithium to neon...
Detailed Explanation
When filling electrons in atoms, a consistent order is followed based on increasing energy. For instance, after the first two shells are filled (1s2 and 2s2), additional electrons go into the 3s and 3p orbitals, maintaining a systematic pattern that can be predicted.
Examples & Analogies
This filling pattern can be visualized like filling bowls with different sizes: you fill the smallest bowl first until it’s full before using the next bigger bowl. As you progress through the elements, you always follow this familiar order.
Stability of Electron Configurations
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ground state electronic configuration of the atom of an element always corresponds to the state of the lowest total electronic energy.
Detailed Explanation
Elements are more stable when their electron configurations are full or half-full. This means configurations like 1s2 2s2 2p6 are crucial because they achieve a stable, lower energy state.
Examples & Analogies
Consider a game where players can only be fully effective if they have the right number of team members. When a team is complete (like a full electron shell), they perform better and are harder to beat—this mirrors how full electron configurations lead to stability in atoms.
Exceptions to Configuration Rules
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In certain elements such as Cu, or Cr, where the two subshells (4s and 3d) differ slightly in their energies, an electron shifts...
Detailed Explanation
Some elements exhibit unexpected electron configurations because stability is enhanced when orbitals are half-filled or fully filled. This is notable in transition metals where energy levels can shift based on electron arrangement.
Examples & Analogies
Think about a sports team where some players require specific positions to maximize teamwork. In the case of copper and chromium, shifting players (electrons) to different roles (orbits) results in stronger team dynamics (greater stability), thus altering expected placements.
Key Concepts
-
Electronic Configuration: The arrangement of electrons in an atom's orbitals.
-
Aufbau Principle: Electrons fill the lowest energy orbitals first.
-
Pauli Exclusion Principle: No two electrons can have the same quantum numbers.
-
Hund's Rule: Electrons occupy degenerate orbitals singly before pairing.
Examples & Applications
The electronic configuration of Neon is 1s² 2s² 2p⁶.
For Sodium, the electronic configuration is 1s² 2s² 2p⁶ 3s¹.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Fill them up, from low to high, electrons fly in their energy sky.
Stories
A brave electron decided to fill a room (orbital) starting from the bottom, bringing friends (other electrons) only after it first settled in.
Memory Tools
To remember the order of filling: '1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p', think 'Silly People Start Putting Down Bunks.'
Acronyms
APH for Aufbau, Pauli, Hund.
Flash Cards
Glossary
- Aufbau Principle
The principle that electrons occupy the lowest energy orbitals first.
- Pauli Exclusion Principle
No two electrons in an atom can have the same set of four quantum numbers.
- Hund's Rule
Electrons occupy degenerate orbitals singly before pairing up.
- Electronic Configuration
The arrangement of electrons in an atom's orbitals.
- Orbital Diagram
A visual representation of an atom's orbitals and the electrons in them.
Reference links
Supplementary resources to enhance your learning experience.
