Thomson Model of Atom
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Atomic Models
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today, we’re starting our journey through atomic models. Starting from the very basic concept, the atom itself was once believed to be indivisible. But what did J.J. Thomson contribute to this idea?

Thomson proposed that atoms had smaller components—like electrons!

Exactly! His model introduced the idea of electrons being embedded in a positive mass. This model is often called the 'plum pudding model.' Can anyone tell me why it’s called that?

Because it resembles a pudding with fruits mixed in!

Great analogy! Now, let’s delve into why this model was significant and its eventual drawbacks.
Thomson's Key Findings
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

What are some key features of the Thomson model that helped explain atomic structure?

The model explained how atoms could be overall neutral despite having charged particles.

Exactly! But with Rutherford's work later on, what flaws were revealed in Thomson's model?

Rutherford’s gold foil experiment showed that the positive charge was not spread out but concentrated in a nucleus.

Well said! Can anyone summarize how this transition from Thomson to Rutherford changed our view on the atom?

It changed from a simple model to a complex one with a nucleus and electrons in well-defined orbits.
Importance of Experimental Evidence
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Why do you think experimental evidence is crucial in science, particularly in atomic theory?

It helps validate or invalidate theories that we create based on observations.

Absolutely! Let’s think back to Rutherford's experiments—what findings of his challenged Thomson’s model?

He found that most alpha particles passed through the foil, meaning there was a lot of empty space in the atom!

Exactly! Understanding the composition of an atom was essential to advance from Thomson's ideas. Can anyone explain why its implications mattered for later atomic theory?

It set the stage for further models that could better explain the behavior of electrons.
Key Transitions in Atomic Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone describe the shifts in understanding that arose from Thomson's and Rutherford's models combined?

After Thomson, we had a better sense of smaller particles like electrons, but Rutherford showed that this positive charge must be centralized, leading to the idea of a nucleus.

Well articulated! This evolution highlights how scientific understanding builds upon previous models and experimental findings. What can this teach us about scientific methods?

We should always question and test our theories to approach closer to the truth.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
J.J. Thomson's atomic model, introduced in 1898, suggested that atoms consist of a spherical mass of positive charge in which electrons are embedded. While it explained the atom's overall neutrality, it was later disproved by Rutherford's experiments, which showed the atom has a dense nucleus.
Detailed
Thomson Model of Atom
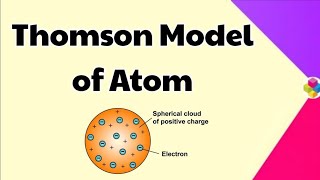
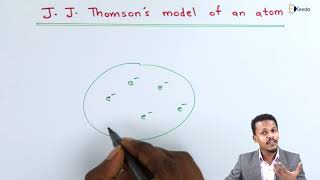
In 1898, J.J. Thomson proposed a model of the atom that represents it as a uniformly distributed sphere of positive charge with negatively charged electrons embedded within it. This model is often colloquially referred to as the plum pudding model due to its resemblance to a dessert where plums (electrons) are embedded in a pudding (the positive charge).
Key Aspects of the Thomson Model:
- Uniform Charge Distribution: The positive charge is spread uniformly over the sphere, resulting in a neutral overall charge for the atom.
- Electrons: The electrons are scattered throughout the positive charge, somewhat like plums in a pudding or seeds in a watermelon.
Significance:
This model essentially shifted the understanding of atomic structure from indivisible atoms proposed by Dalton to a more intricate particle-based system. However, Thomson's model had its limitations, as it could not account for the results of later experimental evidence, notably from Rutherford's gold foil experiment.
Rutherford demonstrated that atoms consist of a small, dense nucleus where most of the atom's mass is concentrated. Consequently, this led to the understanding that the electronic structure is more complex than what Thomson's model could suggest.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Thomson's Proposal
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radius approximately 10–10 m) in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement.
Detailed Explanation
In 1898, J. J. Thomson proposed a new model of the atom that was radically different from prior concepts. He suggested that the atom is a sphere filled with positively charged material, and that electrons are embedded within it. This resembles a pudding with raisins, hence the model is often called the 'plum pudding model.' The concept is that the positive charge balances out the negative charges from the electrons, creating an overall neutral atom. This was a crucial step in understanding atomic structure, but it would later be disproven by later experiments.
Examples & Analogies
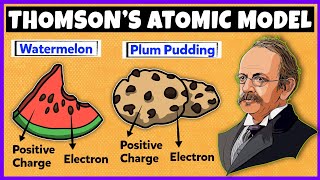
Think of a chocolate chip cookie where the cookie dough represents the positively charged part of the atom and the chocolate chips represent the electrons. Just like the chocolate chips are distributed throughout the cookie, electrons are embedded in the positively charged 'dough' of the atom.
Naming and Characteristics of the Model
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Many different names are given to this model, for example, plum pudding, raisin pudding or watermelon. This model can be visualized as a pudding or watermelon of positive charge with plums or seeds (electrons) embedded into it.
Detailed Explanation
The Thomson Model is also referred to as the 'plum pudding model' because it visualizes the atom as having a uniform mass and positive charge, with electrons (negative charge) scattered throughout. The imagery helps to understand that the positive charge is not localized in one spot but rather spread out uniformly throughout the atom. This model explains the overall neutrality of atoms since the positive charge balances the negative charge from electrons.
Examples & Analogies
Imagine a watermelon where the green outside represents the positive charge of the atom and the seeds represent the electrons. Just like seeds are distributed within the watermelon, electrons are spread within the positively charged space of the atom.
Limitations of the Thomson Model
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Although this model was able to explain the overall neutrality of the atom, but was not consistent with the results of later experiments.
Detailed Explanation
The Thomson model was eventually proven inadequate because it couldn’t account for experimental evidence that pointed to the existence of a dense central nucleus within the atom. Experiments such as Rutherford's gold foil experiment showed that atoms have most of their mass concentrated in a small nucleus and that electrons orbit this nucleus. Therefore, while Thomson's model laid important groundwork for atomic theory, it was ultimately replaced by a better understanding of atomic structure.
Examples & Analogies
If the Thomson model were like a soft ball (where the whole thing is squishy and uniform), the Rutherford model that came later would be like a ping pong ball (where there's a hard core inside). The discoveries made after Thomson's model helped us realize that there’s more to the structure of atoms than just being a uniformly charged object.
Nobel Prize and Legacy
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thomson was awarded Nobel Prize for physics in 1906, for his theoretical and experimental investigations on the conduction of electricity by gases.
Detailed Explanation
Thomson's work was significant because it introduced the idea of subatomic particles and electricity's particulate nature. The Nobel Prize he received reflected the importance of his contributions to science, particularly in our understanding of atomic structure. This laid the foundation for later developments in atomic theory, including the discovery of the nucleus and the electron cloud model.
Examples & Analogies
Just like an inventor’s groundbreaking ideas pave the way for future technologies, Thomson's insights into the nature of atoms initiated a series of discoveries that would ultimately change how we perceive matter, much like the invention of the wheel transformed transportation.
Key Concepts
-
Thomson Model: Atom contains electrons embedded in a positively charged mass.
-
Plum Pudding Model: Concept describing the atom as a sphere of positive charge with negative electrons throughout.
-
Rutherford Experiment: Demonstrated the presence of a dense nucleus at the atom's center.
Examples & Applications
Thomson's plum pudding model illustrates the atom as a distributed mass with embedded electrons.
Rutherford's gold foil experiment contradicted Thomson's model by revealing a concentrated nucleus.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a pudding, electrons hide, Positive charges they abide, Thomson's view once held high, Rutherford watched the changes fly.
Stories
Imagine a giant pudding where each fruit represents an electron. This pudding is held together by a sweet mass, representing positive charge, embodying Thomson's theory before Rutherford revealed the concentrated 'nucleus'!
Memory Tools
Remember 'PRECIOUS' for Thomson's model: Positive charge + Embedded Raisins = Charges Held Uniformly (P.R.E.C.I.O.U.S.).
Acronyms
P.E.T. - Positive Embedded Technology (The concept that describes Thomson's atomic structure characteristics).
Flash Cards
Glossary
- Plum Pudding Model
A historical model of the atom proposed by J.J. Thomson, suggesting that electrons are embedded within a positively charged sphere.
- Nucleus
The small, dense, positively charged center of an atom, discovered by Ernest Rutherford.
- Subatomic Particles
Particles smaller than an atom, including protons, neutrons, and electrons.
Reference links
Supplementary resources to enhance your learning experience.
