A Useful New State Function
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Practical Applications of Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In what practical scenarios do you think enthalpy plays a meaningful role?

In making Chemical Engineering processes more efficient!

Absolutely! Industries constantly rely on enthalpy to optimize reactions, improve efficiencies, and predict yields. Why do you think it’s beneficial to know the change in enthalpy for a particular reaction?

It helps in determining whether a reaction is exothermic or endothermic!

Exactly! Enthalpy changes can tell us the nature of a reaction, whether it absorbs or releases heat. That information can dictate how we proceed with various reactions. Let’s bring this all together with a summary of our discussions.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Enthalpy (H) is defined as the sum of internal energy and pressure-volume work (pV) and serves as a state function to evaluate heat changes in a system at constant pressure. This is particularly relevant for chemical reactions, which typically occur under these conditions.
Detailed
In chemical thermodynamics, reactions commonly occur at constant pressure rather than constant volume. As a result, the need for a new state function arises to describe the heat absorbed or released during these processes. Enthalpy, defined as H = U + pV, where U is internal energy, provides this measure. The change in enthalpy (∆H) corresponds to heat transfer at constant pressure (qp), making it a practical tool for assessing reaction energetics.
Specifically, for a chemical reaction or process, the change in enthalpy can be represented as:
∆H = H2 - H1 = qp
Thus, enthalpy fundamentally reflects the energy variation in a system during reactions, accommodating heat transfer naturally in open systems. Furthermore, this state function operates independently of the reaction pathway, thus providing crucial insights when thermodynamic conditions are analyzed in various phases.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Heat Absorbed at Constant Volume
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
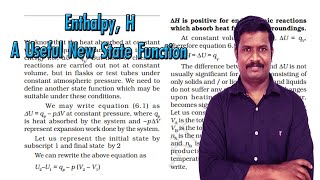
We know that the heat absorbed at constant volume is equal to change in the internal energy i.e., ∆ U = qV. But most of chemical reactions are carried out not at constant volume, but in flasks or test tubes under constant atmospheric pressure.
Detailed Explanation
The first part of this chunk emphasizes that, while the formula ∆U = qV provides a way to calculate heat absorption when volume is kept constant, most chemical reactions occur at constant pressure rather than constant volume. This is important because it influences how we calculate energy changes in these reactions.
Examples & Analogies
Consider boiling water in a pot. The water's volume is effectively constant as it boils, but the atmospheric pressure remains constant. Thus, to measure how much heat is absorbed, we need a different way of thinking—this leads us to the concept of enthalpy.
Defining Enthalpy
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We may write equation (5.1) as ∆U = qp – p∆V at constant pressure, where qp is heat absorbed by the system and – p∆V represent expansion work done by the system.
Detailed Explanation
This chunk incorporates the concept of enthalpy (H). It defines the relationship between internal energy (U) and heat (q) at constant pressure, suggesting that the change in internal energy can be expressed as the heat absorbed minus the work done due to the expansion of the system. Enthalpy helps simplify calculations regarding heat exchange in reactions that occur at constant pressure.
Examples & Analogies
Think about a soda can being shaken and then opened. The pressure inside the can remains constant after it is opened. When you open it, gas escapes, doing work against the atmospheric pressure. The total energy change includes both the heat absorbed by the liquid and the work done during the expansion of gas.
Understanding Enthalpy Change
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Now we can define another thermodynamic function, the enthalpy H as: H = U + pV.
Detailed Explanation
In thermodynamics, enthalpy is defined as the sum of the internal energy of the system (U) and the product of pressure (p) and volume (V). It reflects the total energy of a thermodynamic system that can be used for work and heat transfer, making it a crucial quantity in the study of chemical reactions.
Examples & Analogies
Imagine a car engine. The internal energy includes the energy in the fuel, which is transformed during combustion. The pressure and volume of the gases produced also contribute to the engine's power, thus enthalpy summarizes these two energy components crucial for the engine's operation.
Independence of Enthalpy Change from Path
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Although q is a path dependent function, H is a state function because it depends on U, p, and V, all of which are state functions. Therefore, ∆H is independent of path. Hence, qp is also independent of path.
Detailed Explanation
This section highlights an important feature of enthalpy: it is a state function, meaning its value only depends on the current state of the system (reflected in U, p, and V) rather than how the system reached that state. Conversely, heat (q) is dependent on the specific path taken to achieve that state, which makes calculating enthalpy changes simpler in chemical reactions.
Examples & Analogies
Consider navigating from one city to another. Regardless of whether you take a highway or a back road, the distance between the two cities remains the same. Similarly, the enthalpy change (like the distance) only cares about the starting and ending conditions, not the route taken.
Application of Enthalpy
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For finite changes at constant pressure, we can write equation 5.7 as ∆H = ∆U + p∆V.
Detailed Explanation
This part applies the concept of enthalpy to practical scenarios, specifically for finite changes at constant pressure. The equation illustrates how enthalpy change incorporates both the change in internal energy and the work done during volume expansion or contraction, which is key for calculating energy changes in reactions carried out at constant pressure.
Examples & Analogies
Think of baking a cake. The mixture (system) expands when heated (volume change), and you’re also adding energy (heat) from the oven. The enthalpy change tells you the total energy considered during this baking process.
