Gibbs Energy and Equilibrium
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Gibbs Energy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're diving into Gibbs energy, a crucial concept in thermodynamics. Can anyone tell me what Gibbs energy represents?

Is it related to the energy available for work in a system?

Exactly! Gibbs energy allows us to predict whether a reaction is spontaneous. When Gibbs energy decreases, the process is more likely to occur spontaneously.

What is the formula for Gibbs energy again?

The formula is ΔG = ΔH - TΔS. Here, ΔH is the change in enthalpy, T is the temperature, and ΔS is the change in entropy. Can someone tell me what that means?

It means that the spontaneity of a reaction depends on its enthalpic and entropic changes!

Great job! Remember that a negative ΔG indicates that a reaction is spontaneous.

To help you remember, let's think of ΔG as the 'Go' energy for reactions. If it's positive, it's a 'No Go'!
Understanding Spontaneity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's elaborate on the spontaneity of reactions using Gibbs energy. If ΔG < 0, what does that tell us about the reaction?

It indicates that the reaction can proceed spontaneously!

Correct! Conversely, if ΔG > 0, the process is non-spontaneous. What do you think happens at ΔG = 0?

That's when the system is at equilibrium, right?

Right! When at equilibrium, no net change occurs as the forward and reverse reactions occur at the same rate.

So, Gibbs energy indicates not just if a reaction can happen, but when it will stop changing?

Exactly! Just like a stoplight; it signals when to go and when to stop. Remember, it's also key to predict how much work can be done by the system.
Link Between Gibbs Energy and Equilibrium Constant
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's connect Gibbs energy to equilibrium constants. The relationship is expressed as: ΔG° = -RT ln K. What does this mean?

It means that we can calculate Gibbs energy based on the equilibrium constant of a reaction.

Precisely! And if we know ΔG, we can infer K. This is important for understanding the extent of reactions.

How does temperature affect this relationship?

Great question! Higher temperatures can change the spontaneity of reactions, especially if ΔH is positive. Think of it like increasing the speed on a race track—it can change who wins!

How do we remember these formulas easily?

You can create a mnemonic: 'Go and Stay Happy'—G for Gibbs, S for Spontaneity, and H for Heat or the relationship with enthalpy. Remember it reflects energy balance!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The Gibbs energy (G) is a critical thermodynamic function used to assess the spontaneity of processes at constant temperature and pressure. This section explores how changes in Gibbs energy correlate with enthalpy and entropy, highlighting the conditions for both spontaneous and non-spontaneous reactions. Additionally, it establishes the link between Gibbs energy and the equilibrium constant (K) of reactions.
Detailed
Gibbs Energy and Equilibrium
The Gibbs free energy (G) is an essential thermodynamic function that combines enthalpy (H) and entropy (S) to determine the spontaneity of thermodynamic processes at constant temperature and pressure. The Gibbs energy change (
ΔG) for a reaction can be expressed mathematically as:
ΔG = ΔH - TΔS
This equation indicates that the spontaneity of a process depends not only on the change in enthalpy (ΔH) but also on the change in entropy (ΔS) multiplied by the absolute temperature (T).
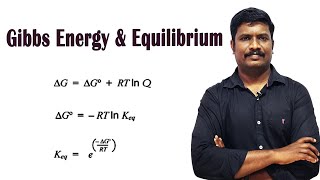
When ΔG is less than zero (ΔG < 0), the process is spontaneous; when ΔG is greater than zero (ΔG > 0), it is non-spontaneous. At equilibrium, ΔG equals zero (ΔG = 0), indicating no net change in the system. Furthermore, there exists a direct relationship between ΔG and the equilibrium constant (K), summarized by the equation:
ΔG° = -RT ln K
where R is the universal gas constant and T is the temperature in Kelvin. This relationship allows chemists to calculate either Gibbs energy changes from known equilibrium constant values or vice versa. Therefore, understanding Gibbs energy is crucial for predicting the feasibility of reactions and the extent of product formation at equilibrium.
Youtube Videos

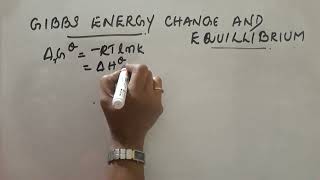






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Free Energy Change and Spontaneity
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We have seen how a knowledge of the sign and magnitude of the free energy change of a chemical reaction allows:
(i) Prediction of the spontaneity of the chemical reaction.
(ii) Prediction of the useful work that could be extracted from it.
Detailed Explanation
This chunk highlights the importance of Gibbs free energy (∆G) in predicting two key aspects of chemical reactions: spontaneity and potential work.
Spontaneity refers to whether a reaction can occur without external influence. When we analyze a chemical reaction, we can calculate free energy changes using the Gibbs free energy equation. If ∆G is negative, the reaction is spontaneous; if it is positive, the reaction is not spontaneous.
Moreover, knowing ∆G helps us estimate how much useful work can be extracted from a reaction under suitable conditions. Thus, Gibbs free energy combines both thermodynamic stability (related to energy) and the direction of reaction (related to entropy) into one informative measure.
Examples & Analogies
Think of free energy like a roller coaster ride. If the ride goes downhill, it feels exciting and happens without much effort (spontaneous). However, if the ride goes uphill, it requires energy to pull the cart (non-spontaneous). Similarly, a negative Gibbs free energy indicates a downhill (spontaneous) process that could do work, just like gravity can do work as it pulls you down a hill.
Reversible Reactions and Equilibrium
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So far we have considered free energy changes in irreversible reactions. Let us now examine the free energy changes in reversible reactions.
‘Reversible’ under strict thermodynamic sense is a special way of carrying out a process such that the system is at all times in perfect equilibrium with its surroundings. When applied to a chemical reaction, the term ‘reversible’ indicates that a given reaction can proceed in either direction simultaneously, so that a dynamic equilibrium is set up.
Detailed Explanation
This section defines what is meant by reversible reactions in a thermodynamic context. Reversible reactions are those that can proceed in both the forward and backward directions, establishing a dynamic equilibrium. At equilibrium, the rates of the forward and reverse reactions are equal, meaning no net change in concentration occurs over time. This indicates that the system is in a state where it has reached a balance concerning the energy among the reactants and products. Reversible reactions are generally more efficient in reaching completion than irreversible ones because they can adjust to changes in conditions until the point of equilibrium is reached.
Examples & Analogies
Imagine a seesaw perfectly balanced in the middle. Both children (representing products and reactants) can go up and down, and the seesaw will stay balanced (equilibrium) unless one child exerts more force (changing conditions). A reversible reaction is like this seesaw: it can tip in either direction, simulating the balance seen in a chemical equilibrium where the rates of opposing reactions are equivalent.
Criteria for Equilibrium
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This means that the reactions in both the directions should proceed with a decrease in free energy, which seems impossible. It is possible only if at equilibrium the free energy of the system is minimum. If it is not, the system would spontaneously change to configuration of lower free energy.
Detailed Explanation
In any reversible reaction at equilibrium, the Gibbs free energy (∆G) reaches a minimum value. This is significant because a system tends to move towards a state of lower free energy, as this represents increased stability. If the free energy is not at its minimum, the system will naturally shift towards minimizing free energy until it reaches equilibrium. Therefore, the state of equilibrium represents a balance, where the Gibbs free energy is at its lowest possible value for the conditions present.
Examples & Analogies
Think of a heavy ball resting at the bottom of a bowl (minimum free energy). If you push it to the side (increase free energy), it will roll back down to the bottom when released (seeking equilibrium). Similarly, reactions that are not at equilibrium will 'roll back' to a state of lower energy until equilibrium is reached.
Gibbs Energy and Equilibrium Constant
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Gibbs energy for a reaction in which all reactants and products are in standard state, ∆rG° is related to the equilibrium constant of the reaction as follows:
0 = ∆rG° + RT ln K
or ∆rG° = – R T ln K
or ∆rG° = – 2.303 RT log K.
Detailed Explanation
This chunk provides the mathematical relationship between Gibbs free energy change (∆G°) and the equilibrium constant (K) of a chemical reaction. Essentially, it presents how the free energy change can be calculated using the equilibrium constant, which summarizes the extent to which a reaction proceeds at equilibrium. A negative value of ∆G° indicates that the reaction is favorable and will more likely proceed toward products, while a positive ∆G° indicates the opposite. Understanding this relationship helps predict how far a reaction will proceed under given conditions.
Examples & Analogies
Consider baking bread. The ingredients represent the reactants, and the finished bread is the product. If you don't follow the right 'recipe' (equilibrium constant), your bread may not rise (positive ∆G°), failing to produce the desired result. But if you measure everything accurately (negative ∆G°), you'll end up with a delicious loaf! The relationship between Gibbs energy and equilibrium constant is like the proportion of ingredients ensuring the success of your baking endeavor.
Key Concepts
-
Gibbs Energy: A crucial measure for spontaneity in chemical reactions.
-
Spontaneity: Determined by the sign of ΔG; negative indicates spontaneous processes.
-
Equilibrium: The state reached when ΔG = 0; where forward and reverse reactions occur at the same rate.
Examples & Applications
In a combustion reaction, ΔG is typically negative, indicating that the reaction can occur spontaneously.
Dissolving salt in water has a negative Gibbs energy change due to the increase in entropy.
The synthesis of ozone (O3) from oxygen (O2) involves a positive enthalpy but can be spontaneous at high temperatures due to the contribution of entropy.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When Gibbs energy goes negative, the change is positive; processes favor it.
Stories
Imagine Gibbs energy as a guide in a forest. If the path is downward (negative ΔG), you can easily flow, but if it's upward (positive ΔG), you're stuck in place.
Memory Tools
Remember 'GEEKS': Gibbs Energy Explains Key Spontaneity.
Acronyms
MICE
Mnemonic for Gibbs = Minimize Internal energy for Constant Entropy.
Flash Cards
Glossary
- Gibbs Energy (G)
A thermodynamic potential that governs the spontaneity of a process at constant temperature and pressure.
- Enthalpy (H)
A measure of total heat content of a system, indicating heat absorbed or released during a process.
- Entropy (S)
A measure of disorder or randomness in a system, often associated with the distribution of energy.
- Equilibrium Constant (K)
A numerical value that represents the ratio of concentrations of products to reactants at equilibrium at a given temperature.
Reference links
Supplementary resources to enhance your learning experience.
