Lattice Enthalpy
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Lattice Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to explore lattice enthalpy. Can anyone tell me what they think lattice enthalpy refers to?

Is it about the energy related to ionic compounds?

Exactly! Lattice enthalpy refers to the energy change when one mole of ionic compound forms from its gaseous ions.

So, does it mean energy is released or absorbed?

Great question! It can either be released in exothermic reactions or absorbed in endothermic ones. Let's remember this with the acronym 'FAB': Forming Always Benefits energy release.

Now, can someone explain why it’s important to understand this concept?

I think it's important because it helps us understand solubility and stability of ionic compounds.

Exactly! The lattice enthalpy gives us insights into these properties.

In summary, lattice enthalpy is crucial for understanding the behavior of ionic compounds, particularly how readily they form and dissolve.
The Born-Haber Cycle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's dive deeper into how we can calculate lattice enthalpy. One common method is the Born-Haber cycle. Who can tell me what this cycle involves?

Is it the series of steps to get the lattice enthalpy?

Exactly! The Born-Haber cycle breaks the formation process into several steps, making it possible to indirectly find the lattice enthalpy.

What are some of those steps?

Good question! It includes sublimation of the solid, ionization of the metal, and the enthalpy of formation of the gaseous ions. Let's remember this sequence with the mnemonic 'SIE: Solid to Ion Energy'.

Why can’t we measure lattice enthalpy directly?

Direct measurement is challenging due to the high energy changes involved and the instability of gaseous ions under standard conditions. That's where indirect methods like the Born-Haber cycle become vital.

Let’s summarize: The Born-Haber cycle allows us to harness the steps of ionic formation to efficiently calculate lattice enthalpy.
Practical Applications of Lattice Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's wrap up our discussion by talking about the practical implications of lattice enthalpy. How does it affect the solubility of ionic compounds?

If the lattice enthalpy is too high, it would be less soluble in water, right?

Exactly! When the energy needed to break the ionic lattice is greater than the energy released when the ions are solvated, the compound remains insoluble.

Can you give an example of such a compound?

Sure! Compounds like barium sulfate or some fluorides are insoluble due to their high lattice enthalpy.

What about ionic compounds with lower lattice enthalpy?

Good point! They tend to be more soluble, such as sodium chloride. The balance of these energies determines the solubility of the compound.

In summary, lattice enthalpy is a crucial factor that influences both the solubility and the stability of ionic compounds.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the concept of lattice enthalpy, explaining how it represents the energy associated with the formation of ionic compounds from gaseous ions. It outlines the significance of lattice enthalpy in thermodynamics, particularly in relation to the stability and solubility of ionic compounds.
Detailed
Lattice Enthalpy
Lattice enthalpy is a critical concept in thermodynamics that describes the energy change when one mole of an ionic compound is formed from its gaseous ions. This value can be either an exothermic or an endothermic process, depending on whether energy is released or absorbed during the formation of the ionic lattice.
The lattice enthalpy can be quantified experimentally or theoretically, often requiring indirect methods such as the Born-Haber cycle, because direct measurement is usually impractical. Lattice enthalpies provide insights into the stability of ionic compounds; smaller values indicate weaker interactions within the ionic lattice, leading to greater solubility in solvents like water.
In describing examples such as sodium chloride, NaCl, the lattice enthalpy is observed as the enthalpy change during the transition from solid state to gaseous ions. The calculations derived from lattice enthalpy are paramount for understanding the thermodynamic processes involving solubility and ionic reactions.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Lattice Enthalpy
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The lattice enthalpy of an ionic compound is the enthalpy change which occurs when one mole of an ionic compound dissociates into its ions in gaseous state.
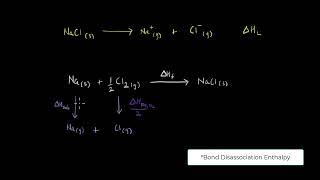
NaCl(s) → Na⁺(g) + Cl⁻(g); ∆latticeH⁰ = +788 kJ mol⁻¹.
Detailed Explanation
Lattice enthalpy describes the energy required to separate one mole of an ionic solid into its ions in the gas phase. For example, when sodium chloride (NaCl) is broken apart into sodium ions (Na⁺) and chloride ions (Cl⁻), it requires energy, resulting in a positive lattice enthalpy value, such as +788 kJ/mol for NaCl.
Examples & Analogies
Think of lattice enthalpy like pulling apart a magnet that’s been stuck to a metal surface. Just as it takes energy to pull the magnet away (which might be difficult if the magnet is strong), it similarly takes energy to separate the ions in a crystalline salt like NaCl.
Determining Lattice Enthalpy Indirectly
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
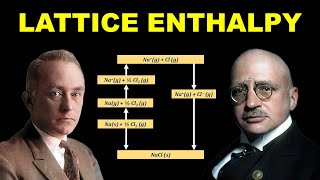
Since it is impossible to determine lattice enthalpies directly by experiment, we use an indirect method where we construct an enthalpy diagram called a Born-Haber cycle.
Detailed Explanation
The Born-Haber cycle is a thermodynamic cycle that shows how to calculate the lattice enthalpy using measured quantities. This involves various steps including sublimation of the metal, ionization of atoms, and the formation of gaseous ions. The lattice enthalpy can then be inferred as the sum of these steps.
Examples & Analogies
Consider it like planning a road trip where you can’t directly measure the distance between two points but know the distances of various legs of the journey. You can add those distances to get the total distance. Here, the individual steps of the Born-Haber cycle are the ‘legs’ of the journey to calculate the lattice enthalpy.
Example of Calculating Lattice Enthalpy
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let us now calculate the lattice enthalpy of Na+Cl−(s) by following steps given below :
1. Na(s) → Na(g) (sublimation of sodium metal, ∆subH⁰ = 108.4 kJ mol⁻¹)
2. Na(g) → Na⁺(g) (ionization of sodium atoms, ionization enthalpy ∆iH⁰ = 496 kJ mol⁻¹)
3. ½Cl2(g) → Cl(g) (dissociation of chlorine, this reaction enthalpy is half the bond dissociation enthalpy, ½∆bondH⁰ = 121 kJ mol⁻¹)
4. Cl(g) → Cl⁻(g) (electron gained by chlorine atoms, ∆egH⁰ = −348.6 kJ mol⁻¹).
Detailed Explanation
To find the lattice enthalpy of sodium chloride, we take the following steps, where each step represents a change in energy:
1. Sublimation is turning solid sodium into gas, which requires energy.
2. Next, ionization involves removing an electron from the gaseous sodium atom, which also requires energy.
3. Then we break the bond in gaseous chlorine to form individual chlorine atoms, using half the energy of the bond dissociation.
4. Finally, when a chlorine atom gains an electron to form a chloride ion, energy is released. The total lattice enthalpy can then be calculated using these values in a Born-Haber cycle by adding and subtracting them accordingly.
Examples & Analogies
Imagine baking a cake. First, you gather the ingredients (sublimation), then you mix them (ionization), next you prepare some of the components (dissociation of chlorine), and finally, you put everything together to bake it (electron gain). Just like you combine the ingredients in various steps, you compute the lattice enthalpy step-by-step using energy changes.
Lattice Enthalpy and Solubility Relations
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The enthalpy of solution of AB(s), ∆solH⁰, in water is, therefore, determined by the selective values of the lattice enthalpy, ∆latticeH⁰, and enthalpy of hydration of ions, ∆hydH⁰ as ∆sol H⁰ = ∆latticeH⁰ + ∆hydH⁰.
Detailed Explanation
The lattice enthalpy is an essential factor because it tells us how strong the ionic bonds are in a solid. When that solid dissolves in water, the energy involved in overcoming these bonds (lattice enthalpy) and the energy released when ions are surrounded by water (hydration enthalpy) must be considered. The overall enthalpy of solution indicates whether the solid will dissolve or not.
Examples & Analogies
Think of lattice and hydration enthalpy like climbing a hill and then coming down. The effort you put into climbing (lattice enthalpy) needs to be weighed against the enjoyment when you slide down and enjoy the view (hydration enthalpy). The final decision of whether the journey is worth it (overall solubility) depends on the net energy gained or lost through this process.
Key Concepts
-
Lattice Enthalpy: The energy needed to separate one mole of an ionic solid into gaseous ions.
-
Formation of Ionic Compounds: Understanding the stability influenced by lattice enthalpy.
-
Born-Haber Cycle: A systematic way to calculate lattice enthalpies indirectly.
Examples & Applications
The lattice enthalpy of NaCl is used when calculating its stability and solubility in solvents.
Sodium bromide has a lower lattice enthalpy compared to sodium chloride, making it more soluble.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Lattice bonds tight, energy must fight, to break apart, from solid to light.
Stories
Imagine an ionic castle made of salt, where the ions hold hands tight. Breaking them apart takes energy much like a knight needs strength to pull apart a gate.
Memory Tools
Lattice - Always Measure The Energies (LAME) to remember the process of measuring lattice enthalpy.
Acronyms
LE - Lattice Energy, measures the energy required to separate ionic solids.
Flash Cards
Glossary
- Lattice Enthalpy
The enthalpy change occurring when one mole of an ionic compound dissociates into its gaseous ions.
- Exothermic Reaction
A reaction that releases energy in the form of heat.
- Endothermic Reaction
A reaction that absorbs energy from its surroundings.
- BornHaber Cycle
An indirect method to calculate lattice enthalpy by breaking down the formation process into steps.
- Solubility
The ability of a substance to dissolve in a solvent.
Reference links
Supplementary resources to enhance your learning experience.
