Spontaneity
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Spontaneity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are diving into the concept of spontaneity. What do we mean when we say a process is spontaneous?

I think it means the process can happen on its own, right?

Exactly! Spontaneous processes occur without needing energy from an external source. For example, when a gas expands to fill a vacuum.

So, does that mean all natural processes are spontaneous?

Not quite. While many natural processes are spontaneous, some reactions can occur only with energy input, like heat moving from a colder to hotter body. Does anyone know why?

Is it because of the direction of heat flow?

Good point! Heat flows naturally from hot to cold, not the other way, unless we do work on the system. That leads us into discussing entropy!

Entropy is about disorder, right?

Correct! Entropy measures the amount of disorder or randomness in a system. We'll explore how it relates to spontaneity next.
Entropy and Spontaneity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's dive deeper into entropy. Why do we think it's important for spontaneous processes?

Because a higher level of disorder means more randomness, so the system trends towards that?

Exactly! The second law of thermodynamics states that the total entropy of an isolated system will either increase or remain the same. Can you think of examples where entropy increases?

Mixing two different gases together increases the entropy of the system!

Also, when ice melts, it goes from a solid state to a liquid state, which is more disordered.

Great examples! Entropy is a driving force for spontaneous processes. But remember, we also need to consider enthalpy—let’s talk about how they interact.
Relationship Between Enthalpy and Gibbs Free Energy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We’ve talked about entropy; now let’s connect that to enthalpy through Gibbs free energy. Can anyone recall the Gibbs equation?

"Isn’t it
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Spontaneity refers to processes that occur without external influence. This section examines how changes in enthalpy and entropy relate to spontaneous reactions, highlighting that while a decrease in enthalpy can indicate spontaneity, it’s the increase in total entropy that primarily governs whether a reaction will occur spontaneously. The Gibbs free energy equation further integrates these two concepts to predict spontaneity.
Detailed
Spontaneity
Overview
In thermodynamics, spontaneity refers to the ability of a process to occur on its own, without external assistance. This section provides a comprehensive analysis of the factors that influence spontaneous processes, primarily focusing on the concepts of entropy, enthalpy, and Gibbs free energy.
Key Points
- Spontaneous Processes: These are reactions that can occur without external energy. For instance, the expansion of a gas into a vacuum is spontaneous due to its natural tendency to occupy all available space.
- Heat Flow Direction: Spontaneous processes, such as heat transfer, occur in one direction—from higher to lower temperature. Non-spontaneous processes, such as heat flowing from a cooler to a warmer body, do not happen without external work.
-
Entropic Consideration: The notion of entropy (
S
), a measure of disorder, is crucial in determining spontaneity. Generally, the total entropy of an isolated system tends to increase, suggesting a natural direction towards disorder. -
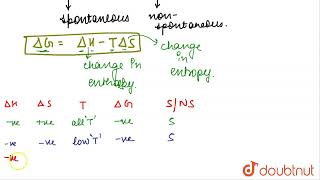
Gibbs Free Energy: Defined as
G = H - TS
, where
H
is enthalpy and
T
is temperature. The change in Gibbs free energy (
ΔG
) is a major determinant of spontaneity: if
ΔG < 0
, the process is spontaneous. - Relation Between ΔH and ΔS: Although a decrease in enthalpy often favors spontaneity, there are endothermic reactions (with positive ΔH) that are spontaneous due to sufficiently large increases in entropy.
Conclusion
Understanding spontaneity is essential for chemical thermodynamics, as it helps predict the feasibility of reactions based on their enthalpy and entropy changes. The use of Gibbs free energy unifies these concepts, offering a clear criterion for spontaneous processes.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Spontaneity
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The first law of thermodynamics tells us about the relationship between the heat absorbed and the work performed on or by a system. It puts no restrictions on the direction of heat flow. However, the flow of heat is unidirectional from higher temperature to lower temperature. In fact, all naturally occurring processes whether chemical or physical will tend to proceed spontaneously in one direction only.
Detailed Explanation
This chunk explains that the first law of thermodynamics does not dictate how heat flows in terms of direction but establishes that heat naturally flows from hotter to cooler bodies. Spontaneous processes are those that occur naturally without external intervention, typically moving from a state of higher energy or order to lower energy or disorder.
Examples & Analogies
A good analogy is to think about ice melting. If you leave a piece of ice on a counter, it will melt spontaneously without any help. It's a natural process that moves from the ordered arrangement of ice molecules (solid state) to a more disordered arrangement in water (liquid state).
Characteristics of Spontaneous Processes
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A spontaneous process is an irreversible process and may only be reversed by some external agency.
Detailed Explanation
This chunk outlines that spontaneous processes tend to be irreversible, meaning they cannot return to their original state without external intervention. For example, if you burn wood, it turns into ash and smoke, and you cannot simply revert it back to wood; you would need to supply energy and materials to recreate the wood from the ash.
Examples & Analogies
Consider how a broken egg cannot spontaneously come back together and turn back into an intact egg. It is a clear example of an irreversible process—once the egg is broken, it cannot return to its original state without significant external work.
Enthalpy and Spontaneity
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If we examine the phenomenon like flow of water downhill or fall of a stone to the ground, we find that there is a net decrease in potential energy in the direction of change. By analogy, we may be tempted to state that a chemical reaction is spontaneous in a given direction, because decrease in energy has taken place, as in the case of exothermic reactions.
Detailed Explanation
This chunk explains that in spontaneous processes, there’s often a decrease in energy, analogous to how objects fall or flow downwards, decreasing their potential energy. However, this is too simplistic. Some processes that have increasing enthalpy can still occur spontaneously, indicating that other factors must also be considered.
Examples & Analogies
Think about rolling a ball down a hill. The ball speeds up and the potential energy decreases as it rolls down. Similarly, many chemical reactions release energy (like combustion) and are spontaneous for that reason. However, just because a reaction absorbs energy doesn't mean it can't be spontaneous. Stories of volcanoes erupting come to mind: the buildup of magma can lead to explosive eruptions, which demonstrate spontaneity despite the inherent energy necessary to begin that change.
Entropy's Role in Spontaneity
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In an isolated system, there is always a tendency for the system's energy to become more disordered or chaotic and this could be a criterion for spontaneous change!
Detailed Explanation
This chunk introduces the concept of entropy as a measure of disorder in a system. The principle of entropy states that systems naturally progress towards states of higher disorder. This chaos or disorder tends to increase as spontaneous processes occur—this is a vital factor determining spontaneity alongside changes in enthalpy.
Examples & Analogies
Imagine a box that is separated into two compartments containing different types of colored balls. If the barrier is removed, the balls will spread out and become mixed. Over time, the arrangement of balls becomes more disordered, thus illustrating an increase in entropy. The initial state of organization is less stable compared to the mixed state, which reflects our real-world experience of spontaneity toward disordered states.
Gibbs Free Energy
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For a spontaneous change, ∆G is negative (< 0), the process is spontaneous.
Detailed Explanation
This chunk summarizes how Gibbs free energy (∆G) is critical in determining whether a process is spontaneous. A negative Gibbs free energy change indicates that the process can occur spontaneously at constant temperature and pressure. This ties together changes in both enthalpy and entropy to evaluate spontaneity.
Examples & Analogies
Think of baking a cake. The ingredients (like flour, sugar, and eggs) mix and undergo a chemical reaction when heated, producing a cake (the end product). The process is spontaneous because it has a negative Gibbs free energy change, meaning that the product (the cake) is chemically more stable than the reactants, reflecting the driving force behind the process.
Entropy and the Second Law of Thermodynamics
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The second law of thermodynamics explains why spontaneous exothermic reactions are so common.
Detailed Explanation
This chunk points out that the second law of thermodynamics states that in any spontaneous process, the total entropy of an isolated system will always increase. This law helps us understand why many exothermic reactions (those that release heat) are spontaneous since the entropy of the surroundings increases as energy spread out.
Examples & Analogies
Consider a candle burning in a room. The heat and light from the burning candle increase the disorder in the surrounding air, causing the surrounding environment to gain energy and entropy. This is a practical illustration of how spontaneous processes increase overall entropy.
The Third Law of Thermodynamics
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The entropy of any pure crystalline substance approaches zero as the temperature approaches absolute zero.
Detailed Explanation
This chunk introduces the third law of thermodynamics, which claims that at absolute zero (0 Kelvin), a perfect crystal would have zero entropy. This is because, at absolute zero, particles are in their lowest energy state and perfectly ordered. As the temperature rises above zero, entropy increases due to molecular motion.
Examples & Analogies
A relatable analogy can be iceberg formations. At temperatures well above freezing, icebergs can break apart and drift, representing high entropy, whereas at absolute zero, the ice would be immobile and perfectly structured, demonstrating zero entropy.
Key Concepts
-
Spontaneity: Refers to processes that occur naturally without external force.
-
Entropy: A thermodynamic property indicating the level of disorder within a system.
-
Gibbs Free Energy: A crucial function that combines the system's enthalpy and entropy to predict spontaneity.
Examples & Applications
The combustion reaction of hydrocarbons is spontaneous; it releases energy.
Ice melting into water is an example of increasing entropy, as the ordered solid becomes a liquid.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Gibbs free energy tells the way, spontaneous processes here to stay.
Stories
Imagine a hill, with a ball on top, it rolls down on its own; that's spontaneous, no stop!
Memory Tools
Remember 'GHS': Gibbs, Heat, Spontaneity.
Acronyms
Use 'SAGE' for Spontaneity
Spontaneous
At
Gibbs energy
Entropy.
Flash Cards
Glossary
- Spontaneity
The ability of a process to occur without external influence.
- Entropy (S)
A measure of the disorder or randomness of a system.
- Gibbs Free Energy (G)
A thermodynamic potential that measures the maximum reversible work performed by a system at constant temperature and pressure.
Reference links
Supplementary resources to enhance your learning experience.
