Hess's Law of Constant Heat Summation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Hess's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will discuss Hess's Law, a fundamental concept in thermodynamics. Can someone tell me what they think this law might indicate?

I think it might have something to do with how heat changes during a reaction.

Great! Hess's Law does deal with enthalpy changes during reactions. It states that if a chemical reaction can occur in multiple steps, the total enthalpy change is the sum of the enthalpy changes for each step. This means that the overall energy change depends only on the initial and final states, not the path taken. Hence, we say enthalpy is a state function.

Can you give an example of how this might work?

Sure! For instance, if we wanted to know the enthalpy change for the reaction of carbon to form carbon dioxide, but it can't be measured directly, we can use related reactions that involve carbon. This allows us to apply Hess's Law effectively.

So, are you saying I can calculate the enthalpy change by adding up the changes of these related reactions?

Exactly, Student_3! That’s the essence of Hess's Law. To recap: Hess's Law allows us to calculate total enthalpy changes through sums of smaller, individual reactions.
Examples of Hess's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's look at an example to apply Hess’s Law. Consider this reaction: C(s) + O2(g) → CO2(g); what do you think its enthalpy change is?

I have no idea! How would we find out?

Good question! We might look at two steps. First, C(s) + O2(g) → CO(g) and then CO(g) + 1/2 O2(g) → CO2(g). If we know the enthalpy changes for these two steps, we could sum them to find the enthalpy for our original reaction.

So, if one of those reactions had an enthalpy change of -283 kJ and the other -393.5 kJ, we would add those up?

Exactly! Applying Hess’s Law lets us manage complicated reactions effectively. This way we don’t need to directly measure every reaction's enthalpy, making it a powerful tool in thermodynamics.

Now I see! It's like putting together pieces of a puzzle.

Yes, exactly! Understanding how we can combine these reactions helps us bring clarity to thermodynamic calculations.
Application of Hess's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss why Hess’s Law is so useful. Can anyone think of an implication in chemistry or industry?

Maybe it helps when reactions are hard to measure, like in labs or industry settings?

Exactly! In many cases, direct measurements of enthalpy changes are not feasible. Hess’s Law allows chemists to work around this limitation.

This is also useful in calculating the energy production of fuels, right?

Right! Calculating the enthalpy change of combustion fuels is a prime application of Hess's Law. Understanding the energy released or required in chemical reactions is fundamental in energy management and production.

So, by applying this law, you can design better energy systems!

Absolutely right, Student_3! Using Hess’s Law enables better design and efficiency in chemical processes and energy systems, leading to both environmental and economic benefits.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Hess’s Law is a fundamental principle in thermodynamics, asserting that the total enthalpy change of a reaction can be calculated using the sum of enthalpies of multiple steps leading to that reaction. This property highlights enthalpy as a state function and reinforces the idea that the route taken by the system does not impact the total energy change.
Detailed
Hess’s Law of Constant Heat Summation
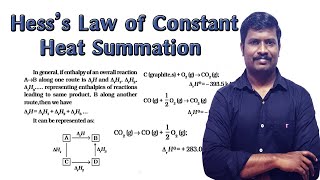
Hess’s Law states that in any chemical reaction, if it can be expressed as the sum of simpler reactions, the enthalpy change for the overall reaction is equal to the sum of the enthalpy changes for each of the individual simpler reactions, regardless of the number of steps taken in the reaction. This law is crucial in thermodynamics as it illustrates that enthalpy is a state function. This means that its change is determined only by the initial and final states of the system, not the path taken to achieve that change. Therefore, Hess’s Law enables chemists to calculate enthalpy changes for reactions that may be difficult to measure directly by using the enthalpy changes of related reactions. For example, if direct measurement of the enthalpy change of a reaction producing a specific product is impractical, but several intermediate reactions leading to that product are known, Hess’s Law can still be applied to determine the total enthalpy change for the desired reaction.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Hess’s Law
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We know that enthalpy is a state function, therefore the change in enthalpy is independent of the path between initial state (reactants) and final state (products). In other words, enthalpy change for a reaction is the same whether it occurs in one step or in a series of steps.
Detailed Explanation
Hess’s Law states that the total enthalpy change during a chemical reaction is the same, regardless of whether the reaction occurs in one step or multiple steps. This means if you know the enthalpy changes for processes that lead to the same final product, you can add those values together to find the total enthalpy change for the overall reaction. This law is based on the principle of conservation of energy, showing that total energy in a closed system remains constant.
Examples & Analogies
Think of it like cooking a meal. You can prepare a dish in one go or by breaking it down into multiple steps. For example, making a cake can be done in a single recipe or by first baking the sponge and then preparing a frosting separately. No matter which method you choose, the total effort (enthalpy change) needed to complete the cake remains the same.
Practical Application of Hess’s Law
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If a reaction takes place in several steps then its standard reaction enthalpy is the sum of the standard enthalpies of the intermediate reactions into which the overall reaction may be divided at the same temperature.
Detailed Explanation
To practically apply Hess’s Law, one can break down a complex reaction into simpler intermediate reactions whose enthalpy changes are known. By summing these individual enthalpy changes, you arrive at the overall enthalpy change for the complex reaction. This is particularly useful in calculating the enthalpy change for reactions that are difficult to measure directly.
Examples & Analogies
Consider a road trip that can be taken via multiple routes. The total distance traveled does not change, regardless of which specific roads you take. If you know the distance of each leg of your trip, you can add them together to find out how far you traveled in total.
Example of Hess’s Law in Action
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let us understand the importance of this law with the help of an example. Consider the enthalpy change for the reaction C (graphite, s) + O2 (g) → CO (g); ∆r H = ? Although CO(g) is the major product, some CO2 gas is always produced in this reaction.
Detailed Explanation
In this example, we can use Harris's Law to find the enthalpy change for the formation of CO from carbon and oxygen by looking at related reactions. Instead of measuring the enthalpy change for the formation of CO directly, we can take the enthalpy of formation of CO2 and the enthalpy change of a reaction involving CO. By manipulating the equations and adding them together correctly, we can deduce the enthalpy change for the desired reaction.
Examples & Analogies
Imagine you want to know how much energy it took to build a bike. Instead of figuring that out directly from the bike, you look at the costs of assembling various parts like the wheels and frame. By summing their assembly costs, you can indirectly determine the total cost of building the bike.
Key Concepts
-
Hess's Law: The summation of enthalpy changes is equal to the total enthalpy change for the reaction.
-
State Function: Quantity dependent only on the initial and final states, not on the path.
-
Enthalpy of Formation: The change in heat content when forming a compound from its elements.
-
Thermochemical Equation: A balanced equation showing the enthalpy change of a reaction.
Examples & Applications
If a reaction C(graphite) + O2(g) → CO2(g) cannot be directly measured, but the steps C + O2 → CO with dH1 and CO + 1/2O2 → CO2 with dH2 are known, Hess's Law allows us to calculate total dH = dH1 + dH2 for the original reaction.
Consider combustion reactions: If we know the enthalpy of CO2 formation and CO's combustion, we can find the enthalpy of carbon combustion indirectly using Hess's Law.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hess’s Law shines bright, change stays the same, for reactions in steps, it's all in the game.
Stories
Imagine walking a path through a forest: no matter how you stroll, the distance from start to end (enthalpy change) remains constant.
Memory Tools
Use the acronym H.E.S.S. to remember: H - Heat, E - Energy, S - State function, S - Sum together!
Acronyms
HESS stands for Heat Energy State Sum.
Flash Cards
Glossary
- Hess’s Law
A principle stating that the total enthalpy change for a reaction is the same, regardless of whether the reaction occurs in one step or multiple steps.
- Enthalpy Change
The amount of heat absorbed or released during a chemical reaction at constant pressure.
- State Function
A property of a system that depends only on its current state, not on the path taken to reach that state.
- Chemical Reaction
A process that leads to the transformation of one set of chemical substances to another.
Reference links
Supplementary resources to enhance your learning experience.
