Enthalpy, H
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're exploring enthalpy, a crucial concept in thermodynamics. Can anyone tell me what we understand by enthalpy?

Is it related to heat and energy in a system?

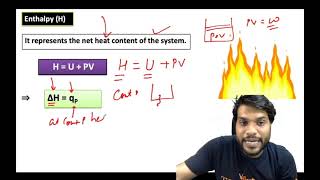
Exactly! Enthalpy is defined as the total heat content of a system at constant pressure. It's denoted as H. Remember this equation: H = U + pV. Can anyone explain what each term means?

U is the internal energy, and p and V are pressure and volume respectively.

Great job! Now, how is enthalpy practically used?

It helps us calculate heat transfer during chemical reactions.

Correct! Since most reactions occur at constant pressure, we often use the change in enthalpy, noted as ∆H, to describe heat exchanges in these reactions.

So, are reactions that have a negative ∆H exothermic?

Yes, exactly! Reactions releasing heat have a negative enthalpy change, while those that absorb heat are endothermic with a positive ∆H.

To summarize, enthalpy is crucial for understanding heat transfer in reactions, especially under constant pressure conditions.
Understanding ∆H and Heat Transfer
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive deeper into how we calculate heat changes using enthalpy. What do we get when we look at the concept of ∆H?

We’re looking at the heat absorbed or released during a reaction, right?

Absolutely! More precisely, it’s the heat transferred at constant pressure. Features like whether the reaction is exothermic or endothermic depend on the sign of ∆H.

Could you provide an example of how we might calculate ∆H?

A common method is through Hess's Law, which states we can sum the enthalpy changes of individual steps in a reaction. So if we have multiple reactions that lead to the same products, we can add their enthalpies to find the total change.

Are there other types of enthalpy changes we need to know about?

Good question! Yes, there’s enthalpy of formation, combustion, and others related to phase changes. Each serves specific purposes in thermodynamic calculations.

To sum up, enthalpy changes are pivotal in determining the heat exchanged in reactions, and knowing how to calculate them greatly helps predict reaction behavior.
Applications of Enthalpy in Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How about we explore some applications of enthalpy? Why is it essential for industries and experiments?

I think it’s about knowing how much energy we need for reactions, especially in manufacturing processes.

Exactly! Industries rely on enthalpy for energy calculations to optimize processes and ensure efficiency. Moreover, it allows us to control reaction temperatures and predict yields.

Does this mean knowing enthalpy can also help in safety?

Yes! By understanding heat releases or absorptions, we can mitigate risks in chemical reactions.

What about reactions that do not produce heat changes?

A valid point! Even if there's no heat change, the enthalpy values still help predict spontaneity and keep reactions within safe energy limits.

As a summary, enthalpy is not just a theoretical concept; it's crucial for practical applications that influence energy management, safety, and efficiency in various industries.
Phase Changes and Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, I want to focus on how enthalpy relates to phase changes. Who can explain what happens during a phase change?

In phase changes, like melting or vaporization, heat is absorbed or released at constant temperature.

Correct! This means we can calculate enthalpy changes related to phase changes using specific enthalpy values assigned for fusion and vaporization.

So, does it mean that enthalpy increases for endothermic phase changes?

Absolutely! For example, melting ice requires heat, thereby absorbing energy and increasing enthalpy. Conversely, freezing releases heat and thus has a negative ∆H.

What are some practical examples of phase changes affecting enthalpy in real life?

Great question! Consider weather phenomena like snow melting or clouds forming. Both involve phase changes that significantly affect enthalpy and local energy cycles.

In summary, understanding the relationship between enthalpy and phase changes aids in grasping how energy transfer influences physical processes.
Review and Synthesis of Enthalpy Concepts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we wrap up our discussion on enthalpy, let's review what we've covered. What is the significance of enthalpy?

It’s important for calculating energy changes in chemical reactions!

Correct! Enthalpy allows us to understand heat transfer during reactions and is crucial for energy management in industries.

Is it true that we often deal with positive or negative enthalpy changes, depending on whether energy is absorbed or released?

Exactly! And we use Hess's Law to find total enthalpy change from multiple contributing reactions.

We also learned about phase changes and the crucial role of enthalpy in processes like melting and vaporization.

Right again! Lastly, remember that enthalpy is not only theoretical but practical—it governs many real-life applications in various fields.

To conclude, today’s insights into enthalpy reinforce its foundational role in thermodynamics and its essential applications across industries.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section introduces enthalpy (H), defining it as a thermodynamic state function related to internal energy, pressure, and volume. It highlights the significance of enthalpy changes in chemical reactions and how these changes are measured. The importance of using enthalpy under constant pressure conditions is emphasized, as it applies to most chemical reactions in practical scenarios.
Detailed
Detailed Summary of Enthalpy
Enthalpy (H) is defined as a thermodynamic state function that combines internal energy (U), pressure (p), and volume (V) of a system into the equation:
H = U + pV
This signifies that the enthalpy of a system reflects its capacity to perform work under constant pressure conditions, which is typical in chemical reactions. The key takeaway is that under constant pressure, the change in enthalpy (∆H) can be equated to the heat absorbed or released by the system (qp). This is expressed in the equation:
∆H = ∆U + p∆V
The section elucidates how using enthalpy is advantageous as it allows for simpler calculations in real-world chemical processes. Through its relationship with heat transfer, enthalpy provides insights into reaction spontaneity and energy efficiency. Furthermore, the chapter also outlines other types of enthalpy changes (like standard enthalpy of reaction and phase transitions) and introduces Hess's law, which is vital for calculating enthalpy changes for multi-step reactions by summing the enthalpy changes of each step.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Enthalpy
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We know that the heat absorbed at constant volume is equal to change in the internal energy i.e., ∆ U = qV. But most of chemical reactions are carried out not at constant volume, but in flasks or test tubes under constant atmospheric pressure. We need to define another state function which may be suitable under these conditions.
Detailed Explanation
Enthalpy is a thermodynamic property that reflects the total heat content of a system at constant pressure. While the change in internal energy (∆U) is directly related to heat at constant volume, many chemical reactions occur under constant pressure conditions, necessitating the definition of enthalpy (H). Thus, we define enthalpy to better describe heat changes in reactions performed at atmospheric pressure.
Examples & Analogies
Imagine cooking a meal: when you heat a pot of soup on the stove, the heat you apply affects the whole pot, not just the water it contains. Similarly, in thermodynamics, we look at energy changes considering the entire system (the pot), not just the isolated parts.
Defining Enthalpy
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
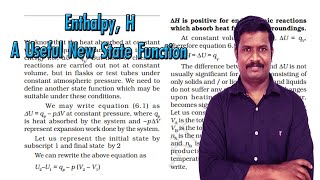
We may write equation (5.1) as ∆U = qp – p∆V at constant pressure, where qp is heat absorbed by the system and – p∆V represent expansion work done by the system. Let us represent the initial state by subscript 1 and final state by 2.
Detailed Explanation
This equation breaks down the relationship between internal energy, heat, and work when a system undergoes changes at constant pressure. The term ∆U represents the change in internal energy, qp is the heat added to the system, and p∆V reflects the work done due to volume changes. This understanding is essential as it allows us to consider both the heat absorbed and the work done in calculating how energy changes during a reaction.
Examples & Analogies
Think of blowing up a balloon: as you blow air into it, the balloon expands (doing work) while you also add energy in the form of the air (heat). The relationship established in the equation helps us understand the overall 'energy' impact on the balloon as you increase its size.
Calculating Enthalpy Change
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Now we can define another thermodynamic function, the enthalpy H [Greek word enthalpien, to warm or heat content] as : H = U + pV, so equation (5.6) becomes qp = H2 – H1 = ∆H.
Detailed Explanation
Here, we define enthalpy as a function that accumulates both internal energy and the energy associated with the pressure and volume of the system. This formula allows us to calculate the heat exchanged in a process at constant pressure, commonly used in chemical reactions. By calculating the difference in enthalpy between the final state (H2) and the initial state (H1), we can determine the change in enthalpy (∆H).
Examples & Analogies
Consider heating water in a kettle: the total energy required to raise the temperature of the water includes not only the internal energy needed to increase its temperature but also the energy associated with the expansion of the steam produced (pV work). This total energy helps quantify the overall energy change during the heating process.
Enthalpy as a State Function
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Although q is a path dependent function, H is a state function because it depends on U, p and V, all of which are state functions. Therefore, ∆H is independent of path.
Detailed Explanation
Being a state function means that enthalpy (H) depends only on the current state of the system and not on how it arrived at that state. This is significant because it simplifies calculations in thermodynamics. It allows us to assess energy changes merely by knowing the initial and final states, without considering the details of the transition itself.
Examples & Analogies
Imagine driving from one city to another; regardless of the route you take, your arrival at the destination (final state) remains the same. Similarly, in thermodynamics, the change in enthalpy (∆H) becomes consistent regardless of the specific details of the process undertaken to reach that state.
Significance of Enthalpy
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is important to note that when heat is absorbed by the system at constant pressure, we are actually measuring changes in the enthalpy.
Detailed Explanation
When a chemical reaction absorbs heat under constant pressure conditions, it is indicative of a change in enthalpy. Understanding this relationship is crucial for predicting how much heat will be released or absorbed, which helps in designing chemical reactions and industrial processes effectively.
Examples & Analogies
Think of a hot air balloon: as the air inside gets heated (absorbing energy), it expands, causing the balloon to rise. By understanding the enthalpy changes involved in heating the air, we can better predict how high the balloon will go as it gains energy.
Key Concepts
-
Enthalpy (H): A measure of total heat content in a system at constant pressure, covering its internal energy, pressure, and volume.
-
Change in Enthalpy (∆H): The heat absorbed or released in a reaction under constant pressure, indicative of thermal changes.
-
Exothermic and Endothermic Reactions: Differentiation based on whether the heat is released or absorbed, thus affecting the sign of ∆H.
-
Hess's Law: The summation of individual enthalpy changes in a sequence of reactions providing the total enthalpy for the final reaction.
Examples & Applications
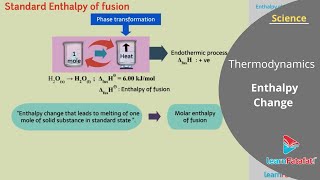
Example 1: When ice melts at 0°C, it absorbs heat and hence is an endothermic reaction with a positive ∆H.
Example 2: The combustion of methane releases heat and is an exothermic reaction with a negative ∆H.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Entalphy's the heat you see, measure it right, the key to chemistry!
Stories
Imagine a party where friends are exchanging gifts—some give away gifts (exothermic, negative ∆H), while others are receiving (endothermic, positive ∆H). This helps remember how heat flows in reactions!
Memory Tools
HESS - Heat is Sum of several Steps, reminding us of Hess's Law.
Acronyms
A mnemonic to remember H = U + pV
'Hugs Us Profoundly Vast' - Enthalpy combines these elements!
Flash Cards
Glossary
- Enthalpy
A thermodynamic state function defined as the sum of internal energy and the product of pressure and volume (H = U + pV).
- ∆H
The change in enthalpy, indicative of the heat absorbed or released during a chemical reaction at constant pressure.
- Exothermic Reaction
A reaction that releases heat, resulting in a negative ∆H.
- Endothermic Reaction
A reaction that absorbs heat, resulting in a positive ∆H.
- Hess's Law
A principle that states the total enthalpy change in a reaction is the sum of the enthalpy changes of individual steps of the reaction.
- Standard Enthalpy of Formation
The change in enthalpy when one mole of a compound is formed from its elements in their standard states.
- Phase Change
A physical transition of a substance from one state of matter to another, such as solid to liquid.
Reference links
Supplementary resources to enhance your learning experience.
