Measurement of ∆U and ∆H: Calorimetry
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Calorimetry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn about calorimetry, a vital technique used in thermodynamics to measure energy changes in chemical reactions. Can anyone tell me why measuring energy change is essential in chemistry?

It's important to know how much energy is released or absorbed during a reaction.

Exactly! Understanding energy changes helps us comprehend reaction spontaneity and thermodynamic properties. Now, let’s discuss how we measure these changes.

What tools do we use for calorimetry?

We typically use a device called a calorimeter. There are different types of calorimeters designed to measure energy changes under varying conditions. Can anyone think of the two main types we'll discuss?

A bomb calorimeter and a regular calorimeter, right?

Correct! A bomb calorimeter is used for constant volume measurements, while a calorimeter for constant pressure is used for measuring enthalpy. Let's take a closer look at how each one functions.
Using a Bomb Calorimeter
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

A bomb calorimeter measures the heat change (∆U) in a system at constant volume. Let's walk through how it works. Who can explain what happens when a substance is combustible?

When the substance is ignited, it burns completely, and the heat released heats the surrounding water.

Good point! The temperature change of the water is recorded. Suppose the calorimeter's heat capacity is known; how can we find the internal energy change?

We can calculate it using the formula q = C ∆T, where C is the heat capacity and ∆T is the change in temperature.

Precisely! So remember, ∆U equals the negative of the heat gained by the water, which will tell us the change in internal energy. Let’s summarize: what key points about bomb calorimetry must we remember?

We measure at constant volume, and q is calculated by knowing the calorimeter's heat capacity.
Measuring Enthalpy Changes with Calorimetry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's move on to measuring enthalpy changes (∆H). Can anyone recall what conditions we measure enthalpy under?

At constant pressure.

That's right! In a standard calorimeter setup, during an exothermic reaction, what happens to qp?

qp will be negative because the system loses heat to the surroundings.

Exactly! Conversely, in an endothermic reaction, qp is positive since the system gains heat. Can anyone tell me how the enthalpy change is calculated?

It’s calculated directly from measuring heat exchanges at constant pressure.

Great! Always remember that qp is equal to ∆H. In summary, we use calorimeters for both constant volume to measure ∆U and constant pressure for ∆H—each critical for understanding energy changes.
Practical Applications of Calorimetry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about some real-world applications of calorimetry. Why do you think this technique is important in industries?

It's useful for determining energy outputs in reactions, which is essential for efficient production.

Absolutely! Industries must understand energy consumption and generation. In what other fields do we utilize calorimetry?

In food science, to calculate the caloric value of foods.

Correct! Calorimetry provides crucial data in nutrition science. Can anyone think of how it might affect environmental studies?

By monitoring reactions that produce greenhouse gases, we can assess their environmental impact.

Right, this shows how essential calorimetry is in various fields. Always think of the broader applications of these principles!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The measurement of energy changes associated with chemical reactions is essential in thermodynamics, with calorimetry being a key technique. This section outlines how ∆U and ∆H can be measured using specific calorimetric methods under different conditions, emphasizing the significance of understanding heat transfer in these processes.
Detailed
Measurement of ∆U and ∆H: Calorimetry
Calorimetry is an experimental technique used to measure energy changes associated with physical and chemical processes. This section focuses on two key aspects: the measurement of internal energy change (∆U) and enthalpy change (∆H). The section explains how calorimetry works, utilizing a calorimeter in which processes are performed in a known liquid volume.
- ∆U Measurements:
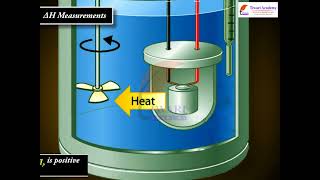
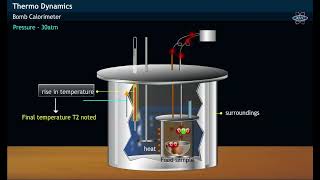
- Heat absorbed at constant volume can be measured using a bomb calorimeter, where a reaction is carried out within a sealed chamber ensuring no volume change occurs. The heat evolved during the reaction raises the temperature of the surrounding water, and the temperature change can be correlated to the internal energy change.
- ∆H Measurements:
- In contrast, enthalpy changes are measured under constant pressure in a standard calorimeter setup. Here, the heat change (qp) is directly related to the enthalpy of the reaction. The section discusses how endothermic and exothermic reactions can be identified based on whether qp is positive or negative.
Overall, understanding the principles of calorimetry is crucial for quantifying energy changes in thermodynamic processes.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Calorimetry
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We can measure energy changes associated with chemical or physical processes by an experimental technique called calorimetry. In calorimetry, the process is carried out in a vessel called calorimeter, which is immersed in a known volume of a liquid. Knowing the heat capacity of the liquid in which the calorimeter is immersed and the heat capacity of calorimeter, it is possible to determine the heat evolved in the process by measuring temperature changes. Measurements are made under two different conditions: i) at constant volume, qV ii) at constant pressure, qp.
Detailed Explanation
Calorimetry is a method used to measure the heat involved in chemical reactions or physical changes. A calorimeter is a device that helps us monitor these changes. It works by immersing a calorimeter in a liquid that has a known heat capacity. This allows us to measure any temperature changes that occur when a reaction happens, helping us quantify how much energy is either absorbed or released by the system in the form of heat. We commonly perform these measurements either at a constant volume (where the volume doesn't change) or at constant pressure (like in most real-life scenarios).
Examples & Analogies
Imagine boiling water to make a cup of tea. You place a pot on the stove (the calorimeter) filled with water (the liquid). As you heat the pot, it absorbs energy from the stove, causing the temperature of the water to rise. By measuring how much the temperature increases and knowing the amount of water, you can calculate how much energy was used to heat the water, just like calorimetry helps scientists measure energy changes in reactions.
∆U Measurements
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For chemical reactions, heat absorbed at constant volume is measured in a bomb calorimeter. Here, a steel vessel (the bomb) is immersed in a water bath. The whole device is called calorimeter. The steel vessel is immersed in water bath to ensure that no heat is lost to the surroundings. A combustible substance is burnt in pure dioxygen supplied in the steel bomb. Heat evolved during the reaction is transferred to the water around the bomb and its temperature is monitored. Since the bomb calorimeter is sealed, its volume does not change; i.e., the energy changes associated with reactions are measured at constant volume. Under these conditions, no work is done as the reaction is carried out at constant volume in the bomb calorimeter. Even for reactions involving gases, there is no work done as ∆V = 0. Temperature change of the calorimeter produced by the completed reaction is then converted to qV, by using the known heat capacity of the calorimeter with the help of equation.
Detailed Explanation
In a bomb calorimeter, we measure the heat energy changes during a reaction at constant volume. This apparatus consists of a steel container that holds the reacting materials and is submerged in water to maintain an accurate reading of energy transfer. When a combustible substance is burned in the calorimeter, the energy released is absorbed by the surrounding water, which raises its temperature. By measuring this temperature change and knowing the heat capacity of the system, we can quantify the heat released during the chemical reaction, using the equation qV. Notably, during this reaction, the volume remains unchanged; therefore, the work done (∆V) is zero, simplifying our calculations.
Examples & Analogies
Consider a pressure cooker that can retain heat without allowing steam to escape, similar to a bomb calorimeter. When food is cooked inside, it uses heat (energy) to change the state of the food without any steam escaping. Just like how the calorimeter measures heat transfer without losing energy to the surroundings, the pressure cooker effectively keeps the heat within, making the cooking process efficient.
∆H Measurements
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Measurement of heat change at constant pressure (generally under atmospheric pressure) can be done in a calorimeter. We know that ∆H = qp (at constant p) and, therefore, heat absorbed or evolved, qp at constant pressure is also called the heat of reaction or enthalpy of reaction, ∆rH.
Detailed Explanation
To measure the heat change at constant pressure, we utilize a calorimeter designed for that purpose, often referred to as a constant-pressure calorimeter. In this scenario, we measure the heat absorbed or released during a chemical reaction, known as enthalpy change (∆H). The relationship here signifies that at constant pressure, the heat exchanged with the surroundings (q) is equivalent to the change in enthalpy (∆H). This is an important aspect of thermodynamic principles, allowing chemists to find out how heat affects reactions under natural atmospheric conditions.
Examples & Analogies
Think about making soup in a pot over the stove. The heat from the stove keeps the pressure inside the pot constant while the soup simmers. During cooking, the soup absorbs heat, which increases its temperature and changes its state as it cooks - this is similar to measuring enthalpy change in a calorimeter. By tasting the soup at different times, you can actually feel the heat change, just as the calorimeter measures it.
Key Concepts
-
Calorimetry: A method to measure energy changes in reactions.
-
Internal Energy (∆U): The total energy of a system.
-
Enthalpy (∆H): The heat content at constant pressure.
-
Types of Calorimeters: Different setups for measuring heat under various conditions.
Examples & Applications
In bomb calorimetry, we can measure the heat released when a fuel combusts.
In a typical calorimeter setup for enthalpy, the temperature change is observed for a reaction occurring in an open container.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Calorimetry, so neat; measures heat without defeat.
Stories
Imagine a scientist using a bomb calorimeter, sealing in their reaction, measuring how much heat is released as they ignite their sample, while water absorbs the warmth.
Memory Tools
For calorimetry, remember C for combustion (bomb calorimeter), and P for pressure (standard calorimeter).
Acronyms
C.U.P. - Calorimetry measures Internal Energy (U) and Enthalpy (P) changes.
Flash Cards
Glossary
- Calorimetry
The experimental technique used to measure heat changes in physical and chemical processes.
- Internal Energy (∆U)
The total energy contained within a system, accounting for all forms of energy.
- Enthalpy (∆H)
The total heat content of a system at constant pressure, defined as the sum of internal energy and the product of pressure and volume.
- Bomb Calorimeter
A device used to measure the heat of combustion of a substance at constant volume.
- Constant Pressure
A condition in which the pressure remains unchanged throughout a process.
- Heat Capacity (C)
The amount of heat required to change the temperature of a given amount of a substance by one degree Celsius.
- Exothermic Reaction
A chemical reaction that releases heat.
- Endothermic Reaction
A chemical reaction that absorbs heat.
- qp
The heat absorbed or released at constant pressure.
Reference links
Supplementary resources to enhance your learning experience.
