Is Decrease in Enthalpy a Criterion for Spontaneity ?
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Spontaneity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're discussing spontaneity in thermodynamic reactions! Who can tell me what spontaneity means?

I think it means a reaction happens on its own without outside help.

Exactly! Spontaneity indicates that a reaction can occur without external intervention. Now, do you think all spontaneous reactions are exothermic?

I thought all spontaneous reactions have to release energy.

That's a common assumption. But today we'll explore that this isn't always true. Some endothermic reactions can also be spontaneous!
Exothermic vs. Endothermic Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's explore further! Exothermic reactions, such as combustion, release heat and usually decrease enthalpy. Can someone give me an example of an exothermic reaction?

Burning wood releases energy, that’s exothermic!

Well done! Now, what about endothermic reactions? How do we feel about them being spontaneous?

I think they might not be spontaneous since they absorb heat.

That’s a fair point, but let's consider examples such as the formation of nitric oxide. Even though it requires heat, it can still be spontaneous!
Role of Entropy in Spontaneity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Here’s a key concept: entropy, denoted as 'S', measures the disorder of a system. When we talk about spontaneous reactions, it’s essential to consider the change in entropy. Who can tell me how entropy influences spontaneity?

If a reaction leads to more disorder, that means higher entropy?

Exactly! For isolated systems, a natural trend is an increase in entropy, indicating a tendency for spontaneous change. Let's look at the diffusion of gases as an example of increasing entropy.

Would that mean that more disordered reactions are usually spontaneous?

Yes! So while a decrease in enthalpy may help, an increase in entropy is a crucial factor for spontaneity.
Entropy and Enthalpy Balance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

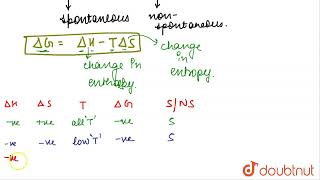
In thermodynamics, we have to consider both enthalpy changes and entropy changes. The Gibbs Free Energy equation is crucial here: G = H - TS. Can anyone explain its significance?

Does that mean if Gibbs Free Energy is negative, the process is spontaneous?

Absolutely! A negative Gibbs Free Energy indicates that the process can occur spontaneously. So now you see how both H and S work together.
Overall Summary and Reflection
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s summarize our discussion today: spontaneity isn't solely determined by enthalpy, but it does play a role. Entropy also greatly impacts whether a reaction can occur. Can anyone summarize what we’ve learned?

So, a reaction can be spontaneous even if it absorbs heat if the increase in disorder is significant?

Exactly! Both enthalpy and entropy must be considered to really understand spontaneity. Remember this while studying reactions in the future!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses spontaneity in chemical reactions, challenging the assumption that a decrease in enthalpy guarantees a reaction is spontaneous. It includes examples of reactions that are endothermic and still spontaneous, introducing entropy as a critical factor in determining spontaneity.
Detailed
Detailed Summary
In the study of thermodynamics, spontaneity refers to whether a reaction occurs without external intervention. A common misconception is that a decrease in enthalpy () alone is a criterion for spontaneity. While exothermic reactions, which release heat and decrease enthalpy, are often spontaneous, many endothermic reactions can also occur spontaneously. Through examples, the section illustrates that reactions like the formation of nitrogen dioxide (N2O4) from nitrogen and oxygen are endothermic yet spontaneous due to increased disorder or chaos in the system.
The concept of entropy, denoted as 'S', quantifies this disorder and acts as a driving force for spontaneity. The section concludes that while a decrease in enthalpy may contribute to spontaneity, it is the total entropy change (S) of the universe that ultimately determines if a reaction can occur spontaneously, providing a clearer criterion for spontaneity.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Concept of Spontaneity
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If we examine the phenomenon like flow of water down hill or fall of a stone on to the ground, we find that there is a net decrease in potential energy in the direction of change. By analogy, we may be tempted to state that a chemical reaction is spontaneous in a given direction, because decrease in energy has taken place, as in the case of exothermic reactions. For example: 1/2 N2(g) + H2(g) = NH3(g); ∆r H = – 46.1 kJ mol–1 1/2 H2(g) + 1/2 Cl2(g) = HCl (g); ∆r H = – 92.32 kJ mol–1 H2(g) + 1/2 O2(g) → H2O(l) ; ∆r H = –285.8 kJ mol–1.
Detailed Explanation
This chunk discusses the concept of spontaneity in chemical reactions and draws an analogy between natural physical processes and chemical reactions. Spontaneous reactions are those that occur without additional energy input, similar to how water flows downhill due to gravity, resulting in a decrease of potential energy. The examples provided showcase exothermic reactions where the enthalpy change (∆H) is negative, indicating a release of energy, reinforcing the idea that a decrease in energy might correlate with spontaneous reactions.
Examples & Analogies
Imagine rolling a ball down a hill; it moves spontaneously due to gravity without any push. Similarly, in chemical reactions like combustion, reactants such as gasoline or wood release energy (in the form of heat) as they transform into products, making the process spontaneous. This resemblance between gravity's role in physical movement and the energy change in exothermic chemical reactions helps us grasp why certain reactions occur naturally and others do not.
Counterexamples: Endothermic Reactions
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Now let us examine the following reactions: 1/2 N2(g) + O2(g) → NO2(g); ∆r H = +33.2 kJ mol–1 C(graphite, s) + 2 S(l) → CS2(l); ∆r H = +128.5 kJ mol–1 These reactions though endothermic, are spontaneous.
Detailed Explanation
In this chunk, we observe that certain spontaneous reactions have positive enthalpy changes, meaning they absorb energy. This contradicts the idea that decreased enthalpy is always necessary for spontaneity. The reactions listed illustrate that despite needing heat to proceed, they can still occur spontaneously under the right conditions. This reveals the complexity in predicting spontaneity based solely on enthalpy.
Examples & Analogies
Consider a cold pack used for injuries. When activated, it absorbs heat from the environment, making its surroundings cooler, but this process happens spontaneously. Just as the pack absorbs energy, these endothermic reactions absorb heat, yet they drive the reaction forward, much like how we can willingly jump into a pool despite the chill, because the experience is refreshing regardless of the cold water.
Understanding Entropy
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Then, what drives the spontaneous process in a given direction? Let us examine such a case in which ∆ H = 0 i.e., there is no change in enthalpy, but still the process is spontaneous. Let us consider diffusion of two gases into each other in a closed container. ... This leads us to entropy, denoted as S.
Detailed Explanation
The chunk transitions to discussing entropy (S) as a key concept in understanding spontaneity. Even when enthalpy does not change, processes can still occur spontaneously, as evidenced in the diffusion of gases. Here, two gases mixing represent a move towards a more disordered state, which aligns with the second law of thermodynamics, stating that the total entropy of an isolated system tends to increase.
Examples & Analogies
Think about how perfume diffuses in a room. Initially, the scent is concentrated in one area, but over time, the fragrance spreads throughout the space. The entropic idea here is that the perfume's molecules are transitioning from an ordered state (concentrated) to a more disordered state (evenly dispersed), a process that occurs spontaneously without any push, similar to spontaneous chemical reactions where energy disperses and order increases.
Conclusion on Enthalpy and Spontaneity
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ultimately, it becomes obvious that while decrease in enthalpy may be a contributory factor for spontaneity, but it is not true for all cases.
Detailed Explanation
This final chunk summarizes the essence of spontaneous processes, asserting that while a decrease in enthalpy can contribute to spontaneity, it is not a definitive criterion across all reactions. The relationship between enthalpy, entropy, and spontaneity requires a nuanced understanding, emphasizing that entropy plays a significant role in these processes.
Examples & Analogies
To cement this idea, consider a hot cup of coffee left on a counter. It will cool down (decrease in energy) and become mixed with the cooler air around it, illustrating enthalpy change. However, the coffee cooling and air mixing do not always define if a reaction or process will happen spontaneously, much like how ice melts in a warm room without external force, where heat flow and entropy contributions dictate the direction of change.
Key Concepts
-
Spontaneity: A reaction that can occur without external assistance.
-
Enthalpy: Key factor in determining heat changes in reactions.
-
Entropy: A measure of disorder, essential for spontaneity considerations.
-
Free Energy: Combines enthalpy and entropy to predict spontaneity.
Examples & Applications
The formation of water can be spontaneous due to the release of energy, indicating a drop in enthalpy.
Some reactions, like the formation of oxygen gas from nitrogen dioxide, are non-spontaneous in the sense of requiring heat yet yield products under certain conditions.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Energy released is great, spontaneity can’t wait!
Stories
Imagine a river flowing downhill—it's effortless. Similarly, reactions naturally tend to occur like that river if they lead to lower energy states or greater disorder.
Memory Tools
Remember G for Gibbs, H for heat, S for spontaneity; G < 0 means it's neat!
Acronyms
G = H - TS
'Good Heat Takes Space' to remember the Gibbs Free Energy equation.
Flash Cards
Glossary
- Spontaneity
The ability of a reaction to occur without external intervention.
- Enthalpy (H)
A measure of heat content in a system.
- Entropy (S)
A measure of disorder or randomness in a system.
- Gibbs Free Energy (G)
A thermodynamic quantity that combines enthalpy and entropy to predict spontaneity.
- Exothermic Reaction
A reaction that releases heat and has a negative change in enthalpy.
- Endothermic Reaction
A reaction that absorbs heat and has a positive change in enthalpy.
Reference links
Supplementary resources to enhance your learning experience.
