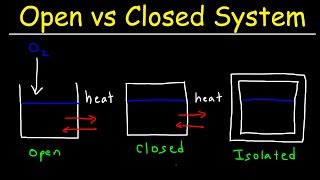
Closed System
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Defining Closed Systems
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing the idea of closed systems in thermodynamics. Can anyone tell me what they think defines a closed system?

Is it a system where no matter can enter or leave, but energy can?

Exactly! In a closed system, we can exchange energy with the surroundings, but the amount of matter stays constant. This contrasts with open systems where both energy and matter can flow in and out.

So, if we have a beaker covered with a lid, would that be a closed system?

That’s right! As long as no substances can escape while heat can move through the lid, it's a closed system. Let's remember this with the acronym 'C.E.M.' - Closed Energy Matter.

What happens to the energy in these systems?

Good question! Energy changes in closed systems can occur through heat transfer or work done by or on the system, which we'll discuss next.
Energy Transfer in Closed Systems
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive deeper into how energy is transferred in closed systems. When energy enters or exits a closed system, what forms can it take?

It could be heat or work, right?

Absolutely! The energy arriving can either be in the form of heat, which is the transfer of thermal energy, or work, which involves the energy transferred through force acting over a distance. Can anyone think of examples for each?

For heat, maybe a hot liquid in a closed thermos?

And for work, it could be when a gas in a piston expands and does work on the piston!

Great examples! Remember, in a closed system, while matter doesn’t enter or exit, energy can change forms, and this lays the foundation for many chemical processes.
Applications of Closed Systems
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss how understanding closed systems aids in practical applications of thermodynamics. Why is it important in terms of chemical reactions?

I think it helps predict the outcomes of reactions within sealed environments, like in engines or batteries.

Exactly! By controlling energy exchanges in a closed system, we can optimize chemical processes efficiently. This principle is extensively applied in industries like energy and materials manufacturing.

So, all chemical reactors could be considered closed systems in many ways?

Yes, many reactors are designed as closed systems to control temperature and pressure, ensuring maximum safety and efficiency in reactions.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Closed systems, contrasted with open and isolated systems, enable energy exchange via heat or work, while preventing mass transfer. This concept is crucial for understanding the principles of thermodynamics.
Detailed
Closed System in Thermodynamics
In thermodynamics, a closed system is defined as one that can exchange energy with its surroundings but not matter. This section emphasizes the importance of understanding system types—open, closed, and isolated—as foundational to the study of energy transformations in thermodynamic processes.
For example, when reactants are placed in a closed vessel, heat can flow in or out of the system, yet the total mass within remains constant. This allows the study of energy changes, which are expressed through the first law of thermodynamics, stating that the internal energy of the system can change only through heat transfer, work, or both.
Understanding closed systems aids in accurately predicting the behavior of chemical reactions in controlled environments, thereby affecting industrial applications and fundamental research in chemistry and physics.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of a Closed System
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In a closed system, there is no exchange of matter, but exchange of energy is possible between system and the surroundings.
Detailed Explanation
A closed system is defined as one where you can have energy interaction with the surroundings (like heat transfer) but no matter can enter or leave the system. This means that while the energy can flow into or out of the system in the form of heat or work, the material (substances) within the system remains constant. An example of a closed system would be a sealed container of gas, where heat can be added or removed but the gas molecules cannot escape.
Examples & Analogies
Think of a pressure cooker. When you heat it, steam (energy) can build up inside, but the water or food inside does not escape. The energy increases, but no matter leaves the pot.
Example of a Closed System
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The presence of reactants in a closed vessel made of conducting material e.g., copper or steel is an example of a closed system.
Detailed Explanation
In a closed vessel made of materials like copper or steel, it allows heat to transfer in and out of the vessel but prevents the contents inside from escaping. This means that any reactions occurring within are contained, and we can measure changes in temperature or pressure resulting from these reactions without losing any of the reactants to the outside world.
Examples & Analogies
Imagine boiling water in a sealed tea kettle. The kettle keeps the water inside (the matter) while steam (energy) can escape through a nozzle once the pressure gets high enough. However, all the water (matter) remains inside the kettle until you pour it out.
Boundaries and Definitions in Closed Systems
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is necessary to think of the system as separated from the surroundings by some sort of wall which may be real or imaginary.
Detailed Explanation
For a closed system, boundaries are crucial as they define what is contained within the system and what is outside of it. These boundaries can either be physical walls (like the walls of a container) or conceptual boundaries (imaginary lines in a larger system). Regardless, these boundaries prevent the exchange of matter while allowing for energy transitions.
Examples & Analogies
Consider a sealed jar. The glass walls separate the contents from the outside air (boundary), preventing any material from getting in or out. However, if you heat the jar, energy can still influence what’s inside, maybe causing pressure to build up, demonstrating energy transfer whilst keeping all the material contained.
Key Concepts
-
Closed System: Defined as a system exchanging only energy, not matter.
-
Energy Transfer: Mechanism through which energy moves as work or heat.
-
Internal Energy: The total energy contained within a closed system.
Examples & Applications
A closed thermos bottle exemplifies a closed system by keeping heat inside while no liquids can escape.
A gas in a piston is a closed system where energy can do work on the piston but matter remains fixed.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a closed system, energy flows, but mass stays in—nobody goes!
Stories
Imagine a sealed jar filled with water and a lid on—it preserves the water but lets heat escape while keeping the liquid inside!
Memory Tools
C.E.M: Closed Energy Matter to remember that in a closed system matter stays, but energy can sway.
Acronyms
C.E.M. - Closed Energy Matter, where only the energy can scatter.
Flash Cards
Glossary
- Closed System
A system that can exchange energy (as heat or work) but not matter with its surroundings.
- Energy Transfer
The movement of energy into or out of a system, which can occur as work or heat.
- Thermodynamics
The study of energy transformations and the laws governing these processes.
Reference links
Supplementary resources to enhance your learning experience.
