Standard Enthalpy of Formation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Standard Enthalpy of Formation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re going to discuss the standard enthalpy of formation, symbolized as ΔfH°. Can anyone tell me what this represents?

Is it the heat change when a compound is formed from its elements?

Excellent! It measures the heat released or absorbed during the formation of one mole of a compound from its elements in their standard states. This is important in calculating the energy changes in reactions. What do we consider as 'standard state'?

I think it's the most stable form of an element at 1 bar and a certain temperature, right?

Yes, typically it's at 25 °C. This definition helps us to compare the enthalpic contributions of different compounds. Remember our acronym 'HEAT' - Heat Energy And Transfer, which summarizes this concept!
Practical Examples
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's look at some examples to understand standard enthalpy of formation better. If I say H2(g) + ½O2(g) → H2O(l); what can we say about its ΔfH°?

I remember it's -285.8 kJ/mol indicating that heat is released when water is formed from hydrogen and oxygen.

Exactly! It's an exothermic reaction. Can anyone mention how we might use these values practically in reactions?

They help in calculating the total heat changes in reactions using Hess's Law, right?

Absolutely! Hess's Law states that the total enthalpy change remains the same irrespective of the pathway taken in reactions. Remember, every value is critical in ensuring accurate energy accounting in chemical processes.
Applications of Enthalpy of Formation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's see how we can apply these values. For example, if we decompose water, what do we know about its formation enthalpy?

It's negative because heat is released when water is formed from its elements!

Great! Given this is exothermic, how would this impact the reverse reaction - the decomposition of water?

That would require heat since it's an endothermic reaction.

Exactly! That required energy aligns with what we know about ΔfH° values. Remember the phrase, ‘Whatever goes up must come down’ - it’s essentially about energy conservation during these transformations.
Deeper Understanding of ΔfH°
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive deeper into calculating ΔfH°. How do we figure out the ΔrH° for complex reactions?

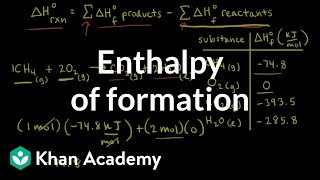
We can use the standard enthalpy change equation: ΔrH° = ΣΔfH°(products) – ΣΔfH°(reactants).

Correct! This equation is crucial for understanding the cumulative energy changes during a reaction. Use the mnemonic 'SUM UP' which stands for 'Standard Underlying math for Unraveling Products'!

So the ΔfH° values tell us how much energy is involved in forming or breaking down substances?

Exactly! Understanding these values is paramount in evaluating thermodynamic stability in reactions.
Recap and Practical Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To conclude, can someone summarize what we've learned about standard enthalpy of formation?

We've learned that ΔfH° quantifies the energy changes when forming a compound from elements under standard conditions.

And that it’s crucial when applying Hess's Law for our calculations in energy changes.

Excellent summaries! Keep in mind that understanding these concepts aids in a profound grasp of thermodynamic principles and their applications in real-world chemistry.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section on standard enthalpy of formation explores the concept of measuring the heat transfer that occurs when one mole of a compound is formed from its constituent elements under standard conditions. This section discusses the significance of these values in thermodynamics, reaction calculations, and energy changes in chemical processes.
Detailed
Overview of Standard Enthalpy of Formation
The standard enthalpy of formation (ΔfH°) is defined as the heat change that occurs when one mole of a compound is formed from its elements in their standard states, which are typically defined as the most stable forms of the elements at 1 bar pressure and a specified temperature, usually 25 °C (298 K).
Significance of Standard Enthalpy of Formation
Standard enthalpy values are crucial for performing calculations related to thermodynamics, such as predicting the heat involved in reactions and calculating the overall enthalpy change for complex reactions using Hess's Law. The values also aid in determining the feasibility of reactions and the stability of compounds.
Examples of Standard Enthalpy of Formation
The typical equations showing standard enthalpy of formation include:
- Water: H2(g) + ½O2(g) → H2O(l); ΔfH° = –285.8 kJ/mol
- Methane: C(graphite) + 2H2(g) → CH4(g); ΔfH° = –74.81 kJ/mol
- Ethanol: 2C(graphite) + 3H2(g) + ½O2(g) → C2H5OH(l); ΔfH° = –277.7 kJ/mol
Conclusion
Understanding standard enthalpy of formation contributes to broader knowledge in thermodynamics and supports the practical applications in chemical synthesis and reaction design.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Standard Enthalpy of Formation
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The standard enthalpy change for the formation of one mole of a compound from its elements in their most stable states.
Detailed Explanation
The standard enthalpy of formation (∆fH°) is defined as the heat change that occurs when one mole of a compound is formed from its constituent elements under standard conditions (1 bar pressure and usually 298 K temperature). This concept is essential in thermodynamics and helps in understanding the energy changes involved in chemical reactions.
Examples & Analogies
Imagine building a house (the compound) using bricks (elements like carbon, hydrogen, and oxygen). The energy required to put together all the bricks into a complete house is analogous to the standard enthalpy of formation. This energy change represents the effort needed to assemble elements into a compound.
Reference States of Elements
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The reference state of an element is its most stable state of aggregation at 25°C and 1 bar pressure.
Detailed Explanation
For the standard enthalpy of formation, it is crucial to define the reference states of elements because the enthalpy changes are measured based on how elements exist in their most stable forms. For example, hydrogen exists as H₂ gas, oxygen as O₂ gas, and carbon in graphite form at standard conditions.
Examples & Analogies
Consider how you might refer to the most common format of items as their 'default setting.' For example, when you describe water, the standard state at room temperature is liquid water, not ice or steam. Just as every compound has a standard state, all elements have a reference state that helps in comparing their energies correctly.
Calculating Standard Enthalpy of Formation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
∆H for the reaction: H₂(g) + ½O₂(g) → H₂O(l); ∆fH° = -285.8 kJ mol–1.
Detailed Explanation
In this equation, we see an example of how the standard enthalpy of formation can be calculated using hypothetical reaction enthalpies. For the reaction that forms water from its elements, the standard enthalpy of formation is given as -285.8 kJ/mol, indicating that the process is exothermic and releases heat.
Examples & Analogies
Imagine cooking food that requires water. When you boil water, it absorbs heat (endothermic), but when water becomes steam (condensation), it releases heat (exothermic). The energy changes in forming water reflect this transition, demonstrating how the formation of chemical bonds can affect energy dynamics in reactions.
Relation to Other Thermochemical Processes
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The standard molar enthalpy of formation (∆fH°) is just a special case of ∆rH°.
Detailed Explanation
The standard molar enthalpy of formation is particularly important when calculating the enthalpy change for various reactions. It represents one specific type of reaction but fits within the broader category of thermodynamic functions that quantify energy changes in chemical processes. Understanding this helps in applying Hess’s law to derive enthalpy changes for entire reaction sequences.
Examples & Analogies
Think of a recipe book where each recipe is an example of a chemical reaction. The standard enthalpy of formation functions like an analogy for individual recipes, while the overall collection of recipes represents the broader knowledge of energy transformations in cooking (or chemistry). Each recipe (reaction) relates back to the foundational ingredients (elements) and their heat contributions (enthalpy).
Key Concepts
-
Standard Enthalpy of Formation: Essential for understanding energy changes in reactions.
-
Exothermic and Endothermic Reactions: Types of reactions defined by heat transfer.
-
Hess's Law: Important for calculating enthalpy changes in complex reactions.
Examples & Applications
The typical equations showing standard enthalpy of formation include:
Water: H2(g) + ½O2(g) → H2O(l); ΔfH° = –285.8 kJ/mol
Methane: C(graphite) + 2H2(g) → CH4(g); ΔfH° = –74.81 kJ/mol
Ethanol: 2C(graphite) + 3H2(g) + ½O2(g) → C2H5OH(l); ΔfH° = –277.7 kJ/mol
Conclusion
Understanding standard enthalpy of formation contributes to broader knowledge in thermodynamics and supports the practical applications in chemical synthesis and reaction design.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Enthalpy hot, heat goes in, formation’s where new compounds begin.
Stories
Imagine a baker forming a cake; each ingredient is an element, combined with care, to create something new. The heat from the oven represents the energy at play.
Memory Tools
Remember 'FROST' - Formation Releasing Of Standard Temperature for ΔfH°.
Acronyms
Use 'SUGAR' - Standard Enthalpy, Units, Generally, Accurate References.
Flash Cards
Glossary
- Standard Enthalpy of Formation (ΔfH°)
The heat change when one mole of a compound is formed from its elements in their standard states.
- Exothermic Reaction
A reaction that releases heat to its surroundings.
- Endothermic Reaction
A reaction that absorbs heat from its surroundings.
- Hess's Law
The total enthalpy change of a reaction is the sum of the enthalpy changes for the individual steps.
- Thermodynamic Stability
The stability of a system in terms of its energy; lower energy states are more stable.
Reference links
Supplementary resources to enhance your learning experience.
