Applications
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Work in Thermodynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to talk about how work is defined in thermodynamics, especially in chemical reactions involving gases. Who can tell me what they think work means in this context?

I think it refers to how energy is transferred when gases expand or compress.

Exactly! The work done by or on a gas can be expressed in the formula w = -p_ext ∆V. Remembering this relationship is important. Anything else about work that comes to mind?

What does `p_ext` mean?

Great question! `p_ext` stands for external pressure. It's crucial to have a handle on how this affects the work in various processes. So, let's explore a bit more.
Types of Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, can someone explain the difference between reversible and irreversible processes?

I think reversible processes are those that can go in both directions without a net change?

Exactly! In a reversible process, we can reverse the changes at any point. In contrast, irreversible processes cannot return to their original state without external intervention. Let's elaborate on why reversibility is important when calculating work done by gases.

Does this mean that we can easily calculate the work in reversible processes since they are well-defined?

Yes, precisely! Reversible processes provide a consistent framework for understanding energy changes, which is beneficial for calculations.
Calculating Work
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's transition into how we can calculate work performed by gases. Can anyone remind me of the ideal gas law?

It's PV = nRT, where P is pressure, V is volume, n is the amount of substance, R is the gas constant, and T is temperature.

Well done! We'll use this law to derive expressions for work done in both isothermal and adiabatic conditions. Who can explain what isothermal means?

It's when the temperature remains constant during a process.

Correct! In an isothermal expansion, the work done can intensively relate to changes in volume at a constant temperature. Let’s do a calculation example together.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains the concept of work in thermodynamics, specifically focusing on pressure-volume work done by gases. It highlights various scenarios including compression and expansion, and introduces the principles of reversible processes and their calculations using the ideal gas law.
Detailed
Applications of Thermodynamics
Thermodynamics plays a crucial role in the study of chemical reactions, particularly regarding how energy changes influence these processes. When considering chemical reactions that involve gases, one of the primary forms of energy transformation is related to work, particularly pressure-volume work, depicted through a system containing an ideal gas within a cylinder fitted with a piston.
In this section, we explore the work done when a gas is compressed or expanded. The work done on the gas during compression is calculated using the formula w = -p_ext ∆V, where p_ext represents external pressure and ∆V is the change in volume. We also discuss conditions under which the external pressure is constant and the work changes accordingly.
Key Concepts:
- Reversible processes: A process is said to be reversible if it occurs in such a manner that system and surroundings remain in near-equilibrium.
- Special cases of work: Different scenarios, such as isothermal and adiabatic expansions, are examined, highlighting their implications on heat exchange and internal energy.
- Relationship between work and gases: Understanding how work interacts with volume changes in gases, particularly in the context of ideal gas behavior, is essential for calculating system energy changes.
The concepts covered here are fundamental to the application of thermodynamics in real-world settings, such as engines and chemical reactors, where the ability to predict energy changes allows for efficient operation and design.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Work Done by a System
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Many chemical reactions involve the generation of gases capable of doing mechanical work or the generation of heat. It is important for us to quantify these changes and relate them to the changes in the internal energy. Let us see how!
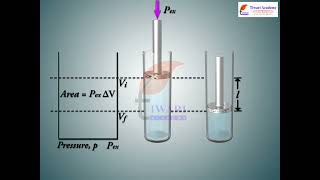
First of all, let us concentrate on the nature of work a system can do. We will consider only mechanical work i.e., pressure-volume work. For understanding pressure-volume work, let us consider a cylinder which contains one mole of an ideal gas fitted with a frictionless piston. Total volume of the gas is V_i and pressure of the gas inside is p. If external pressure is p_ex which is greater than p, piston is moved inward till the pressure inside becomes equal to p_ex. Let this change be achieved in a single step and the final volume be V_f. During this compression, suppose piston moves a distance, l and its cross-sectional area is A.
Detailed Explanation
In this chunk, we begin by discussing how many chemical reactions result in the production of gases that can perform work or generate heat. To better understand this phenomenon, we introduce the concept of mechanical work, specifically focusing on pressure-volume work. We illustrate this with an example involving a cylinder containing an ideal gas and a frictionless piston. Here, the initial volume of the gas is denoted as V_i, and its pressure as p.
When an external pressure (p_ex) greater than the internal pressure of the gas is applied, the piston moves inward, leading to a decrease in the gas's volume (to V_f). Throughout this process, we need to calculate and understand how work is done on the system as the piston compresses the gas.
Examples & Analogies
Think of this system as a bicycle pump. When you compress the pump, you are doing work on the air inside it, similar to how external pressure compresses the gas in our discussion. As you push down on the pump, the air is compressed, increasing its pressure. This example illustrates mechanical work in action, showing how you can relate gas compression to real-world activities.
Calculating Work Done on the System
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If w is the work done on the system by movement of the piston then
w = force × distance = p_ex . A . l = p_ex . (–∆V) = – p_ex ∆V = – p_ex (V_f – V_i).
The negative sign of this expression is required to obtain conventional sign for w, which will be positive. It indicates that in case of compression work is done on the system.
Detailed Explanation
This chunk explains how to calculate the work done on a system during gas compression. The formula provided shows that work (w) is the product of force and distance. In our context, the force exerted on the piston results from the external pressure (p_ex) acting over the piston's cross-sectional area (A) and the distance (l) the piston travels.
In mathematical terms, we can express work as w = p_ex × A × l. This relationship leads to a volume change, represented as ∆V (which equals V_f - V_i). The negative sign in the formula emphasizes that work is done on the system when the volume decreases (compression). Thus, it's essential to understand the conventions around sign when performing thermodynamic calculations.
Examples & Analogies
Imagine yourself pushing a car to start it. By applying force over a certain distance, you're doing work on the car. Similarly, in our gas cylinder, as pressure pushes against the piston's surface, the work done on the gas mirrors the effort required to move the car. In both cases, the work concept is central to understanding how energy transfer occurs.
Reversible Processes in Work Calculation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If the pressure is not constant at every stage of compression, but changes in number of finite steps, work done on the gas will be summed over all the steps and will be equal to −Σ p ∆V.
Detailed Explanation
In this chunk, we explore how to calculate work when pressure isn't constant throughout the compression process. If the external pressure changes in a series of finite steps rather than remaining constant, the total work done on the gas can be found by summing the individual work done during each step. This is indicated mathematically by the expression −Σ p ∆V. This understanding helps to appreciate how in practical scenarios, pressures can fluctuate rather than remain steady, making this form of calculation useful.
Examples & Analogies
Think of a build-up of pressure in a bicycle pump where you apply pressure in bursts. Each push is akin to a step where the pressure within the cylinder varies. By summing the work done during each push, you can assess the total work involved in fully compressing the air, demonstrating the necessity and utility of this calculation method.
Understanding Reversible and Irreversible Processes
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A process or change is said to be reversible if a change is brought out in such a way that the process could, at any moment, be reversed by an infinitesimal change. A reversible process proceeds infinitely slowly by a series of equilibrium states such that the system and the surroundings are always in near equilibrium with each other.
Detailed Explanation
Here, we introduce the concept of reversible processes. A reversible process is one where changes are made gradually, allowing the system to remain in a near-equilibrium state throughout the process. This means that the system can be restored to its initial condition by very small changes. This idealization is important in thermodynamics because it allows for precise work calculations and enables us to describe processes mathematically, optimizing energy efficiency.
Examples & Analogies
Imagine filling a balloon with air very slowly. As you add air gradually, the outside pressure and internal pressure can adjust, keeping everything balanced. If at any point you decide to release air, it can return to its original state just as gradually. This slow, careful process illustrates how we can maintain equilibrium and reversibility, similar to what we find in thermodynamics. Meanwhile, popping the balloon represents an irreversible change where the air can’t return.
Key Concepts
-
Reversible processes: A process is said to be reversible if it occurs in such a manner that system and surroundings remain in near-equilibrium.
-
Special cases of work: Different scenarios, such as isothermal and adiabatic expansions, are examined, highlighting their implications on heat exchange and internal energy.
-
Relationship between work and gases: Understanding how work interacts with volume changes in gases, particularly in the context of ideal gas behavior, is essential for calculating system energy changes.
-
The concepts covered here are fundamental to the application of thermodynamics in real-world settings, such as engines and chemical reactors, where the ability to predict energy changes allows for efficient operation and design.
Examples & Applications
When gas is compressed in a cylinder, the work done on the gas can be calculated using the formula: w = -p_ext ∆V.
In a reversible process, if a gas expands against constant external pressure, the work done can be significant in terms of energy used or produced in the system.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Work on gas, pressure high, volume down, oh my!
Stories
Once there was a gas in a cylinder. When squeezed (compressed), it did work, if let out (expanded), it didn't – a simple tale of pressure and space!
Memory Tools
W = -p∆V: ‘Pressure Varies’ means work done depends on pressure times change in volume.
Acronyms
Remember W = P∆V where W is Work, P is Pressure, and V is Volume to simplify work calculations!
Flash Cards
Glossary
- Work
Energy transferred by a system to its surroundings when it expands or compresses.
- Reversible Process
A process that can be reversed without leaving any net change in the system and surroundings.
- Irreversible Process
A process that cannot return to its original state without external intervention.
- p_ext
The external pressure acting on the gas system.
- Ideal Gas Law
A physical law that describes the behavior of ideal gases in relation to pressure, volume, temperature, and amount.
Reference links
Supplementary resources to enhance your learning experience.
