∆H Measurements
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Calorimetry Basics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome! Today, we'll discuss calorimetry, which is essential for measuring energy changes in reactions. Can anyone tell me what calorimetry is?

Isn't it a way to measure heat changes in chemical reactions?

Exactly! Calorimetry involves using devices called calorimeters. We primarily use two types: bomb calorimeters for constant volume reactions and coffee cup calorimeters for constant pressure. Let’s dive into how these work!

So, what’s the difference between the two?

Great question! In bomb calorimeters, we measure heat during combustion reactions at constant volume, while coffee cup calorimeters are used for reactions in solutions at constant pressure. Let's remember we often denote heat changes with ∆H.

What about exothermic and endothermic reactions?

Good point! In exothermic reactions, ∆H is negative because the reaction releases heat. Conversely, in endothermic reactions, ∆H is positive as they absorb heat. Remember: 'Exo equals exit; heat is lost.' Let's summarize this before we move on.

To conclude: Calorimetry helps us measure energy changes using bomb and coffee cup calorimeters depending on the reaction's nature. Exothermic reactions release heat (negative ∆H), while endothermic reactions absorb heat (positive ∆H).
Understanding Bomb Calorimeters
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s focus on bomb calorimeters. Who knows why they’re used for specific measurements?

Are they used for measuring combustion reactions?

That's right! Bomb calorimeters are crucial for measuring the energy output in combustion reactions, providing accurate readings of heat changes. This is because they operate at constant volume. Can anyone recall what we do with the heat measured?

We use it to determine the ∆U for the reaction?

Exactly! The temperature increase in the surrounding water allows us to calculate the heat evolved, providing insights into the reaction energy. We look at the formula: q = C × ∆T, where 'C' is the calorimeter's heat capacity.

What does that tell us about energy efficiency in reactions?

That's an important aspect! Understanding these measurements can help us optimize conditions for energy efficiency in various chemical processes. Let's summarize what we covered!

In summary, bomb calorimeters allow us to measure the heat released in combustion reactions at constant volume using the equation q = C × ∆T, vital for understanding reaction energetics.
Coffee Cup Calorimetry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s shift our focus to coffee cup calorimeters. Can anyone explain their typical use cases?

I think they're used for reactions involving solutions at constant pressure, right?

Correct! These calorimeters measure the heat change in reactions that occur in liquid solutions, such as acids and bases mixing. During these reactions, the pressure remains constant because they are usually open to the atmosphere.

And how do we distinguish heat changes in these reactions?

Excellent question! When we measure heat changes at constant pressure, we refer to it as qp, and we use it to determine the enthalpy change, ∆H. If qp > 0, the reaction is endothermic, whereas if qp < 0, it’s exothermic.

How is this information practically applied?

It's applied in culinary chemistry, for example, in calculating how much energy is needed for cooking or temperature adjustments in food preparation. Let’s review what we’ve learned.

So, to recap: Coffee cup calorimeters measure heat changes at constant pressure, allowing us to determine the enthalpy of reactions involving solutions. The relationship between qp and ∆H helps in understanding energy dynamics during chemical processes.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides a comprehensive overview of the principles and techniques used to measure enthalpy changes in chemical processes. It focuses on the roles of bomb and coffee cup calorimeters, the significance of constant pressure in measurements, and the interpretation of results to determine whether a reaction is exothermic or endothermic.
Detailed
∆H Measurements in Thermodynamics
Thermodynamics is foundational to understanding energy changes during chemical reactions, particularly through the lens of enthalpy change (∆H). Enthalpy, a crucial thermodynamic state function, allows chemists to describe energy changes associated with heat absorbed or released during a reaction. This section emphasizes measuring these changes under constant pressure, as is common in many practical situations.
Key Concepts Covered
- Calorimetry: The practical measurement of enthalpy changes is conducted using calorimetry. Two key types of calorimeters are introduced:
- Bomb Calorimeter: Used for reactions at constant volume, often in combustion reactions. Here, the heat is transferred to a surrounding water bath, where the temperature change is measured to calculate the energy released during the reaction.
- Coffee Cup Calorimeter: More commonly used for measuring the heat of reactions in aqueous solutions at constant atmospheric pressure.
- Exothermic vs. Endothermic Reactions: Understanding the difference between reactions that release heat (exothermic) and those that absorb heat (endothermic) is critical.
- Exothermic: Negative ∆H, the system loses heat, resulting in an increase in temperature of the surroundings.
- Endothermic: Positive ∆H, the system gains heat, leading to a decrease in temperature around it.
- Significance of the Measurements: Accurate enthalpy measurements help predict whether a reaction can proceed under given conditions and assists in optimizing reaction conditions in practical applications.
Overall, measuring enthalpy changes is vital in thermodynamics, playing a crucial role in understanding chemical reactions, their feasibility, and their applications across various fields.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Experimental Measurement of ∆H
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Measurement of heat change at constant pressure (generally under atmospheric pressure) can be done in a calorimeter shown in Fig. 5.8. We know that ∆H = qp (at constant p) and, therefore, heat absorbed or evolved, qp at constant pressure is also called the heat of reaction or enthalpy of reaction, ∆rH.
Detailed Explanation
In a laboratory setting, the heat change accompanying a reaction conducted at constant pressure is measured using a calorimeter. This apparatus helps to ensure that the heat exchange occurs under controlled conditions. The enthalpy change, denoted as ∆H, is equivalent to the heat exchanged at constant pressure, which is specifically labeled qp. When a reaction releases heat to its surroundings (an exothermic reaction), qp will be negative, indicating that the system is losing heat. Conversely, for endothermic reactions that absorb heat from their surroundings, qp is positive, reflecting the heat gained by the system.
Examples & Analogies
Think of baking a cake. When you mix the ingredients and put them in the oven, heat is absorbed from the oven by the batter (an endothermic process). As the cake bakes, it changes internally due to this heat. If you instead had a process running in your kitchen that released warmth, like grilling meat, the meat would be the system releasing stored chemical energy as heat to the air around it (an exothermic process), similar to how a reaction would produce a negative qp.
Bomb Calorimeter Setup
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
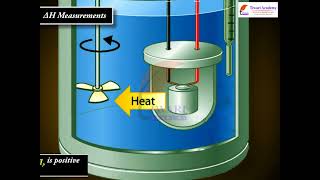
For chemical reactions, heat absorbed at constant volume is measured in a bomb calorimeter. Here, a steel vessel (the bomb) is immersed in a water bath. The whole device is called calorimeter. The steel vessel is immersed in water bath to ensure that no heat is lost to the surroundings.
Detailed Explanation
A bomb calorimeter is an essential tool for measuring the heat transferred during a chemical reaction at constant volume. This device consists of a sealed steel bomb where the reaction takes place, generally under high pressure. By immersing this bomb in a water bath, we can accurately measure the temperature change of the water, which directly correlates to the heat produced or absorbed in the reaction. In this setup, since the volume does not change, we don't take expansion work into account, allowing us to focus solely on the heat change (∆U).
Examples & Analogies
Imagine putting a sealed bag of popcorn in the microwave. The microwave heats the bag, and when the kernels pop, the bag remains sealed, containing all the heat and pressure. Similarly, in a bomb calorimeter, the reactions happen in a sealed environment where heat changes can be precisely measured, just like noticing the temperature of your water mimicking the heat from popcorn expansion.
Exothermic and Endothermic Reactions in Calorimetry
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In an exothermic reaction, heat is evolved, and system loses heat to the surroundings. Therefore, qp will be negative and ∆rH will also be negative. Similarly in an endothermic reaction, heat is absorbed, qp is positive and ∆rH will be positive.
Detailed Explanation
In calorimetry, understanding the type of reaction being studied is crucial. In exothermic reactions, the system reacts to release heat, hence the heat produced leads to an increase in the temperature of the environment (the surrounding water in the calorimeter). When measured, this negative heat exchange indicates a loss of energy from the system, which reflects a negative value for both qp and ∆rH. Conversely, during an endothermic reaction, the system absorbs heat from the surroundings, leading to a decrease in temperature around it. For this case, qp and ∆rH are recorded as positive values since the system is gaining energy.
Examples & Analogies
Think of this like filling up a car with gas. When gas is consumed (exothermic), the car engine releases energy, akin to the warmth you'd feel from a running engine. If your air conditioner is running (endothermic), it's pulling energy from the outside, making the room cooler. Both scenarios reflect how energy is either leaving (exothermic) or entering (endothermic) a system.
Key Concepts
-
Calorimetry: The practical measurement of enthalpy changes is conducted using calorimetry. Two key types of calorimeters are introduced:
-
Bomb Calorimeter: Used for reactions at constant volume, often in combustion reactions. Here, the heat is transferred to a surrounding water bath, where the temperature change is measured to calculate the energy released during the reaction.
-
Coffee Cup Calorimeter: More commonly used for measuring the heat of reactions in aqueous solutions at constant atmospheric pressure.
-
Exothermic vs. Endothermic Reactions: Understanding the difference between reactions that release heat (exothermic) and those that absorb heat (endothermic) is critical.
-
Exothermic: Negative ∆H, the system loses heat, resulting in an increase in temperature of the surroundings.
-
Endothermic: Positive ∆H, the system gains heat, leading to a decrease in temperature around it.
-
Significance of the Measurements: Accurate enthalpy measurements help predict whether a reaction can proceed under given conditions and assists in optimizing reaction conditions in practical applications.
-
Overall, measuring enthalpy changes is vital in thermodynamics, playing a crucial role in understanding chemical reactions, their feasibility, and their applications across various fields.
Examples & Applications
When burning a fuel in a bomb calorimeter, the rise in temperature of the surrounding water helps calculate the energy released during combustion.
Mixing an acid and a base in a coffee cup calorimeter allows scientists to measure the heat evolved or absorbed at constant pressure to determine the enthalpy of the neutralization reaction.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Heat released, feel the beat; Exothermic reactions, can't be beat!
Stories
Imagine you're cooking and the pot gets hot—exothermic! Now picture ice melting in your drink; it's absorbing —endothermic!
Memory Tools
Remember 'During Reactions, Clearly Evaluate Heat'. Use 'DRCHE' to recall that you must evaluate the heat changes during reactions.
Acronyms
HEAT
Heat Evolved or Absorbed Thermodynamically.
Flash Cards
Glossary
- Calorimetry
The science of measuring heat changes in chemical reactions.
- Bomb Calorimeter
A calorimeter designed for measuring the heat of combustion of a substance at constant volume.
- Coffee Cup Calorimeter
A simple calorimeter for measuring heat changes in reactions at constant pressure, typically using an insulated cup.
- Exothermic Reaction
A reaction that releases heat, resulting in a negative change in enthalpy (∆H).
- Endothermic Reaction
A reaction that absorbs heat, resulting in a positive change in enthalpy (∆H).
Reference links
Supplementary resources to enhance your learning experience.
