Enthalpy of Solution
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Basics of Enthalpy of Solution
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’ll explore the enthalpy of solution. Can anyone tell me what happens when a solute dissolves in a solvent?

I think the solute breaks apart and mixes with the solvent!

Exactly! When a solute dissolves, it interacts with solvent molecules. This process involves energy changes. Who remembers the terms for these energy changes?

Is it lattice energy and hydration energy?

Right! The energy required to break the solute’s lattice is lattice energy, and the energy released when solvating is hydration energy. Together, they define the enthalpy of solution.

So, we can write the overall enthalpy change like...?

Good question! It’s expressed as: ∆solH⁰ = ∆latticeH⁰ + ∆hydH⁰. This formula illustrates how the energy required to break bonds can be influenced by the energy released during solvation.

Does that mean if the lattice energy is higher, it would make dissolution harder?

Exactly! Higher lattice energy means more energy is needed to dissolve the solute, often resulting in an enthalpy of solution that’s positive. Let’s summarize briefly: Enthalpy of solution is the heat change involved in the dissolution, which is the sum of lattice enthalpy and hydration energy.
Significance of Lattice and Hydration Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Continuing our discussion, how do hydration and lattice energies affect solubility?

Doesn’t hydration energy help pull the solute into solution?

Correct! Higher hydration energy can overcome lattice energy, promoting solubility. For example, salts with significant hydration energy tend to dissolve better in water than those with high lattice energies.

Could you give us an example?

Sure! Consider sodium chloride. Its lattice energy is balanced well by hydration energy, making it soluble. But lead bromide has high lattice energy, making it less soluble.

Does temperature play a role in this process too?

Absolutely! Higher temperatures often increase the solubility because they provide extra energy that can help overcome the lattice energy barrier. This links back to our earlier discussions. When we see ∆solH⁰ is positive, it often means dissolution requires heat, which is why it can be more effective at higher temperatures.

In summary, we need to consider both types of energies and temperature when discussing solubility?

Spot on! Remember, understanding these concepts helps us predict how substances behave when mixed, which is crucial in chemical processes.
Application of Enthalpy of Solution in Real Life
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we’ve covered the theory, let’s discuss where these concepts apply in real life.

How do we see this in everyday products?

Great question! The enthalpy of solution is fundamental in industries like pharmaceuticals, where solubility can affect drug efficacy.

And what about in food or cooking?

Exactly! When dissolving sugar in hot tea, heat helps the sugar dissolve faster. Here, temperature and solubility become very important!

Can we evaluate solubility based on these principles?

Yes! Using the concepts of lattice and hydration energy allows us to predict solubility patterns. Incorporated in processes, knowing the enthalpy change helps us optimize conditions.

So it’s important beyond just understanding chemistry?

Absolutely! It spans many fields, making it a vital concept to grasp. To wrap up, always connect theory with practical applications.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section covers the enthalpy changes incurred when substances dissolve, the concept of lattice energy and hydration, and how these factors influence solubility and enthalpy of solution. It further explores the role of enthalpy in industrial and biochemical contexts.
Detailed
Detailed Summary
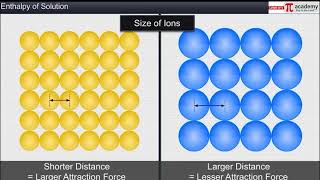
The enthalpy of solution, represented as ∆solH⁰, refers to the heat change observed when one mole of a solute dissolves in a specified amount of solvent at constant temperature and pressure. This process consists of two essential components: the lattice enthalpy, which denotes the energy required to break the ionic lattice of the solute, and the enthalpy of hydration, which describes the energy released when the ions are solvated by the solvent. The overall enthalpy of solution can be expressed as:
∆solH⁰ = ∆latticeH⁰ + ∆hydH⁰
For many ionic compounds, the dissolution process is endothermic due to the high lattice enthalpy, resulting in a net positive enthalpy of solution. However, solubility typically increases with temperature since higher temperatures provide the necessary energy to compensate for the lattice enthalpy. The section emphasizes that while various reactions are exothermic, the endothermic nature of many dissolutions showcases the delicate balance between ∆latticeH⁰ and ∆hydH⁰. Understanding enthalpy changes in solution processes is crucial for predicting reaction behavior and optimizing conditions in various applications, including chemical synthesis and pharmaceuticals.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Enthalpy of Solution
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Enthalpy of solution of a substance is the enthalpy change when one mole of it dissolves in a specified amount of solvent. The enthalpy of solution at infinite dilution is the enthalpy change observed on dissolving the substance in an infinite amount of solvent when the interactions between the ions (or solute molecules) are negligible.
Detailed Explanation
The enthalpy of solution describes the energy change that occurs when a solute dissolves in a solvent. This change can be measured as the difference in energy before and after the solute dissolves. At infinite dilution, the solute makes minimal interactions with the solvent, allowing us to observe the pure enthalpy change of solution without interference from solute-solute interactions.
Examples & Analogies
Think of making a cup of tea. When you add sugar to hot water, it dissolves, and you can notice that the sugar gradually disappears, and the tea tastes sweeter. The energy change involved in this process is similar to the enthalpy of solution. If you could keep adding sugar until you had an unlimited amount of water, you would see how the energy involved would stabilize, capturing the enthalpy of solution at infinite dilution.
Ionic Compounds and Dissolution
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When an ionic compound dissolves in a solvent, the ions leave their ordered positions on the crystal lattice. These are now more free in solution. But solvation of these ions (hydration in case solvent is water) also occurs at the same time.
Detailed Explanation
When ionic compounds, like table salt (NaCl), dissolve in water, the solid's crystal lattice breaks apart. In this process, sodium (Na+) and chloride (Cl-) ions separate and disperse throughout the solution. The water molecules surround these ions, stabilizing them through a process called solvation. Solvation releases energy which can offset the energy required to break the ionic bonds in the solid, leading to the overall energy change (enthalpy of solution).
Examples & Analogies
Imagine a bag of Lego blocks as a crystalline solid representing table salt. When you dump the bag into a pool of water, the blocks spread apart and the water fills the gaps between them. Just like how the Lego blocks lose their arrangement in the bag, the ions in the salt lose their fixed positions, becoming independently surrounded by water molecules.
Enthalpy of Solution Calculation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The enthalpy of solution can be calculated by considering lattice energy and the energy associated with solvation. It can be represented as: ∆sol H = ∆lattice H + ∆hyd H.
Detailed Explanation
To find the enthalpy of solution, we combine two main components: the lattice energy (the energy needed to separate the ions of an ionic compound) and the enthalpy of hydration (the energy released when the ions are surrounded by water molecules). The overall enthalpy change of the solution—whether it feels cold or hot—is the net effect of these two energies, which can be calculated using the provided formula.
Examples & Analogies
Think of ice melting on a hot day. The energy absorbed to melt the ice (lattice energy) is being compensated by the coolness of the surroundings (energy that helps chill the water). Similarly, when you add salt to water, the overall temperature effect (cooling or heating) depends on how much energy is used to break the salt apart versus how much energy is released when the salt ions interact with the water.
Impact on Temperature and Solubility
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For most ionic compounds, the enthalpy of solution is positive, indicating endothermic dissolution, and solubility tends to increase with temperature. However, if the lattice enthalpy is very high, the compound may not dissolve at all.
Detailed Explanation
If the dissolution process absorbs heat (thus having a positive enthalpy of solution), it typically means that the solute requires energy to break apart from its solid form. As temperatures rise, the increased kinetic energy can help the solute dissolve more effectively, which is why many salts dissolve better in hot water. Conversely, if a salt has a very high lattice energy, even heating the solution may not provide enough energy to overcome this barrier for dissolution.
Examples & Analogies
When you attempt to dissolve sugar in boiling water, it dissolves quickly compared to cold water. Picture yourself trying to dissolve rocks in a drink. It doesn’t matter how hot the drink is; if the rock is too dense and requires too much energy to dissolve, it will just sit there while everything else dissolves around it.
Key Concepts
-
Enthalpy of Solution: The heat change when one mole of solute dissolves.
-
Lattice Energy: Energy needed to separate the ions in a solute.
-
Hydration Energy: Energy change involved in solvating ions.
Examples & Applications
Dissolving sodium chloride in water where the hydration energy compensates the lattice energy.
The heat needed to dissolve a salt influences temperature-dependent solubility.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When solutes dissolve, it’s quite the show, Lattice breaks, hydration flows!
Stories
Imagine a sugar cube in hot tea; the heat helps it dissolve, overcoming the lattice formed in solid form, while the taste enhances.
Memory Tools
Lattice energy (LE) first, hydration energy (HE) comes next; remember 'LE He', like 'Let Him dissolve'.
Acronyms
DISH
Dissociation (lattice)
Interaction (hydration)
Solvent
Heat.
Flash Cards
Glossary
- Enthalpy of Solution (∆solH⁰)
The heat change associated with the dissolution of one mole of a solute in a solvent at constant temperature and pressure.
- Lattice Energy
The energy required to separate one mole of an ionic solid into its gaseous ions.
- Hydration Energy
The energy released when ions are surrounded by solvent molecules.
Reference links
Supplementary resources to enhance your learning experience.
