Enthalpies for different types of reactions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are starting with the concept of enthalpy, which is a measure of heat content in a system. Can anyone tell me why enthalpy is important in thermodynamics?

It helps us understand how heat is absorbed or released during chemical reactions.

Exactly! Enthalpy changes are crucial for predicting whether a reaction will occur spontaneously or how much energy will be involved. Remember, enthalpy is represented as H.

Are there different types of enthalpy changes?

Great question! There are several types of enthalpy changes such as the standard enthalpy of combustion, formation, and solution.
Standard Enthalpy of Combustion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s explore standard enthalpy of combustion. This represents the energy released when one mole of a substance combusts completely in oxygen. Can anyone give an example?

The combustion of butane is one example!

Correct! The combustion of butane releases approximately 2658 kJ of energy. This gives us insights into energy production in fuels.

Does this mean we have a high energy release with combustion?

Yes! Combustion reactions are generally exothermic, meaning they release heat, which is useful in applications like internal combustion engines.
Standard Enthalpy of Formation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss the standard enthalpy of formation. It represents the heat change when one mole of a compound is formed from its elements in their standard states. Why is this important?

It helps us to calculate the overall enthalpy change for reactions!

Exactly! We can apply Hess's law, which states that the total enthalpy change is the sum of the changes in a series of reactions. This is powerful for calculating reactions where direct measurement isn’t possible.

Can we have zero enthalpy for something?

Yes! By definition, the standard enthalpy of elements in their standard states is zero.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses the concept of standard enthalpy and its application to various types of reactions such as combustion, formation, and dilution. It highlights the importance of understanding enthalpy changes for predicting reaction behavior and energy transformations during chemical processes.
Detailed
Detailed Summary
This section of the chapter elaborates on the enthalpies associated with different types of reactions, which are crucial for understanding energy changes in thermodynamics. Enthalpy, represented by the symbol H, is a state function that captures heat changes during chemical reactions when they occur at constant pressure.
Key Points Covered:
- Standard Enthalpy of Combustion (ΔcH⁰): Standard enthalpy of combustion is defined as the heat change per mole of a substance during complete combustion under standard conditions. This is typically an exothermic process, releasing energy.
- Example: The complete combustion of butane (C₄H₁₀) releases 2658 kJ of energy.
- Standard Enthalpy of Formation (ΔfH⁰): This refers to the enthalpy change when one mole of a compound is formed from its elements in their standard states. Its value is crucial for calculating reaction enthalpies using Hess's law.
- Note: The standard enthalpy for all elements in their standard states is defined as zero.
- Bond Enthalpy (ΔbondH⁰): This is the energy required to break one mole of bonds in a gaseous substance, influencing the overall energy dynamics during chemical reactions.
- Lattice Enthalpy: This is the enthalpy change associated with the formation of one mole of an ionic compound from its gaseous ions. Understanding lattice enthalpy is important for predicting solubility and the stability of ionic compounds.
- Enthalpy of Solution (ΔsolH⁰): This signifies the enthalpy change when one mole of an ionic compound dissolves in a specified amount of solvent, providing insights into dissolution processes.
Importance in Thermodynamics:
Understanding these different types of enthalpy changes not only helps in the quantitative analysis of chemical reactions but also aids in predicting the feasibility and direction of these reactions based on their energy dynamics. This understanding is crucial for applications in chemistry, industry, and environmental science.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Standard Enthalpy of Combustion
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Combustion reactions are exothermic in nature. These are important in industry, rocketry, and other walks of life. Standard enthalpy of combustion is defined as the enthalpy change per mole (or per unit amount) of a substance, when it undergoes combustion and all the reactants and products being in their standard states at the specified temperature.
Detailed Explanation
Combustion reactions release heat and are crucial in various applications like engines and heating. The standard enthalpy of combustion is measured under standard conditions. This means it considers standard states of all substances involved (reactants and products) at a given temperature, typically 298 K, and 1 bar pressure. This allows us to compare how much energy is released when different fuels burn. For instance, knowing the enthalpy of combustion helps us evaluate different fuels for efficiency or energy output.
Examples & Analogies
Think of a campfire. When wood burns, it releases heat and light; this energy output is akin to what we measure as the standard enthalpy of combustion. The more energy it releases, the more efficient that wood is as a fuel.
Enthalpy of Combustion of Butane and Glucose
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Cooking gas in cylinders contains mostly butane (C₄H₁₀). During complete combustion of one mole of butane, 2658 kJ of heat is released. We can write the thermochemical reactions for this as: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l); ∆Cₕ⁰ = –2658.0 kJ mol⁻¹. Similarly, combustion of glucose gives out 2802.0 kJ/mol of heat.
Detailed Explanation
In these examples, butane and glucose represent common fuels. The heat released during their combustion indicates how much energy can be harnessed for work or heat. For butane, when it burns, it reacts with oxygen to produce carbon dioxide and water, releasing 2658 kJ for each mole. Similarly, carbohydrates like glucose also release substantial energy, crucial for both metabolic processes in living organisms and energy production in industries.
Examples & Analogies
Consider a car engine that runs on butane. When you turn the key and ignite the fuel, it combusts and produces energy that powers the engine. In a similar aspect, our bodies rely on glucose from food. When we consume sugar, our body processes it, releasing energy that we use for daily activities.
Understanding Enthalpy Changes in Reactions
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The enthalpy of combustion of glucose gives out 2802.0 kJ/mol of heat, for which the overall equation is: C₆H₁₂O₆(g) + 6O₂(g) → 6CO₂(g) + 6H₂O(l); ∆Cₕ⁰ = –2802.0 kJ mol⁻¹.
Detailed Explanation
In this example, glucose combines with oxygen during combustion to release energy. The negative sign in the enthalpy change indicates that energy is released, making the reaction exothermic. Understanding these combustion reactions helps us appreciate energy generation from various food sources and fuels, highlighting their importance in both biological systems and the energy economy.
Examples & Analogies
Imagine a candle burning; as it flickers, it transforms wax (a type of fuel) into light and heat. The energy released by the burning candle represents the concept of enthalpy change, much like what happens when glucose or other fuels combust.
Key Concepts
-
Enthalpy: A thermodynamic property that represents heat content in a system.
-
Standard Enthalpy of Combustion: Energy released during the complete combustion of a substance.
-
Standard Enthalpy of Formation: Heat change when one mole of a compound is formed from its elements.
-
Lattice Enthalpy: The heat change when one mole of an ionic compound is formed from gaseous ions.
-
Enthalpy of Solution: The energy change when a substance dissolves in a solvent.
Examples & Applications
Example of Standard Enthalpy of Combustion: The combustion of butane releases 2658 kJ, indicating energy produced during fuel combustion.
Example of Standard Enthalpy of Formation: The formation of water from elemental hydrogen and oxygen results in a specific enthalpy change.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To remember combustion heat, think of fire is neat, it burns with might, releasing light!
Stories
Once upon a time, in a lab filled with flasks, a chemist learned that when butane burns, energy is released and questions are asked.
Memory Tools
Remember HESS - Heat exchanges when Summing Steps for reactions using Hess's Law.
Acronyms
C.F.A. - Combustion, Formation, and Acidic solution changes help recall different enthalpy types.
Flash Cards
Glossary
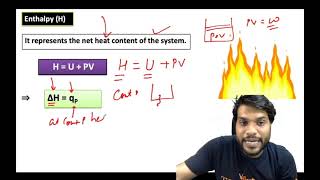
- Enthalpy (H)
A thermodynamic property that represents the total heat content of a system.
- Standard Enthalpy of Combustion (ΔcH⁰)
The enthalpy change when one mole of a substance is completely burned in oxygen under standard conditions.
- Standard Enthalpy of Formation (ΔfH⁰)
The change in enthalpy when one mole of a compound is formed from its elements in their standard state.
- Lattice Enthalpy
The enthalpy change associated with the formation of one mole of an ionic compound from gaseous ions.
- Enthalpy of Solution (ΔsolH⁰)
The heat change when one mole of an ionic compound dissolves in a specified amount of solvent.
Reference links
Supplementary resources to enhance your learning experience.
