Bond Enthalpy
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Bond Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss bond enthalpy. Can anyone tell me what bond enthalpy signifies?

Isn't it the energy needed to break a chemical bond in a molecule?

Exactly right! Bond enthalpy indeed refers to the energy required to break bonds. Know that this information is crucial because it helps us understand how chemical reactions occur.

So, is bond enthalpy different for every type of bond?

Good question! Yes, each bond type has a specific bond enthalpy, which varies between molecules. We use terms like bond dissociation enthalpy and mean bond enthalpy to describe these.

What’s the difference between them?

Bond dissociation enthalpy is about one specific bond, while mean bond enthalpy averages the energy for breaking a particular bond type across similar molecules. This mean value helps us predict the energy changes during reactions.

So the bond energy can influence the stability of a molecule, right?

Absolutely! Higher bond enthalpy means a stronger bond and consequently higher stability. Let's summarize the key points: Bond enthalpy is the energy to break bonds, varies by bond type, and helps predict reaction stability.
Applications of Bond Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's explore how bond enthalpy plays a practical role in thermochemistry. Why do you think it's important for calculating reaction energies?

Because it tells us how much energy we need to put into breaking bonds?

Exactly! When we know the bond enthalpies, we can calculate the total energy for the bonds broken and formed in a reaction. Can someone explain how that might look mathematically?

I think it involves using Hess's Law.

Great connection! We can find the enthalpy change by summing the bond enthalpies of reactants and products. Ready for a practical example?

Sure! What example are we looking at?

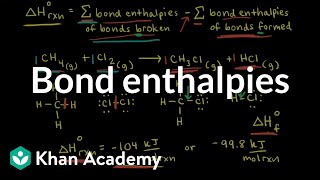
Let's consider methane's combustion: Using the bond enthalpies of bonds in methane and oxygen, we can find the overall energy change and determine if the reaction is exothermic or endothermic.

So it's about relating bond strengths to reaction energies!

Absolutely! Finally, let’s summarize: Bond enthalpy calculation is essential in thermodynamics as it aids in understanding reaction energetics.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Bond enthalpy is a significant concept in thermodynamics that indicates the energy required to dissociate bonds in a molecule. There are two types of bond enthalpy: bond dissociation enthalpy and mean bond enthalpy, both essential for predicting the energy changes during chemical reactions.
Detailed
Bond Enthalpy
Bond enthalpy is the measure of the energy required to break a specific bond in a gaseous molecule. It is vital for understanding thermodynamic processes as it directly relates to the energy changes during chemical reactions. Bond enthalpy can be classified into two primary types:
Bond Dissociation Enthalpy
This is the energy change when one mole of a covalent bond is broken under standard conditions to form gaseous products. For instance, breaking the H-H bond in dihydrogen gas (H2) requires energy, indicating that bond dissociation entails an endothermic process. The bond dissociation enthalpy is specific to each bond type within a molecule.
Mean Bond Enthalpy
For polyatomic molecules, the mean bond enthalpy is taken, which averages the energy required to break a type of bond across various compounds. This average provides a useful reference for estimating the energy changes in reactions involving similar types of bonds.
The significance of bond enthalpy lies in its ability to predict reaction energy changes and stability. By utilizing bond enthalpy values in Hess's law, chemists can calculate the enthalpy changes for reaction pathways based on the bonds broken and formed, thus understanding reaction mechanisms.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Bond Enthalpy
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Chemical reactions involve the breaking and making of chemical bonds. Energy is required to break a bond and energy is released when a bond is formed. It is possible to relate heat of reaction to changes in energy associated with breaking and making of chemical bonds.
Detailed Explanation
Bond enthalpy refers to the energy required to break one mole of bonds in gaseous molecules. To understand this concept, remember that during a chemical reaction, bonds between atoms in molecules are broken, and new bonds are formed as the reaction proceeds. When we say energy is required to break bonds, this means that energy must be supplied to overcome the forces holding atoms together. Conversely, when new bonds form, energy is released into the environment.
Examples & Analogies
Think of bond enthalpy like a rubber band. If you want to stretch (break) the rubber band, you need to pull hard on it until it snaps. This requires effort (energy). Once snapped, if you let the rubber band go, it snaps back to its original shape, releasing the energy stored in it. This is similar to how energy is released when bonds form in a chemical reaction.
Diatomic Molecules and Bond Enthalpy
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consider the following process in which the bonds in one mole of dihydrogen gas (H2) are broken: H2(g) → 2H(g); ∆H–HH = 435.0 kJ mol–1 The enthalpy change involved in this process is the bond dissociation enthalpy of H–H bond. The bond dissociation enthalpy is the change in enthalpy when one mole of covalent bonds of a gaseous covalent compound is broken to form products in the gas phase.
Detailed Explanation
For diatomic molecules like hydrogen gas (H2), the bond enthalpy can be measured as the energy required to break the hydrogen-hydrogen bond to form two hydrogen atoms. This is an essential concept because it quantifies the strength of the bond between the two hydrogen atoms. The value of 435 kJ/mol indicates that 435 kilojoules of energy must be supplied to break one mole of H2 molecules into two moles of H atoms.
Examples & Analogies
Imagine stretching a tightly-held string between two people. The effort it takes to pull the string apart can be likened to the energy needed to break the bond. The tighter (stronger) the string is held, the more effort you’ll need to pull it apart, just like with bond enthalpy.
Polyatomic Molecules and Variability of Bond Enthalpy
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In the case of polyatomic molecules, bond dissociation enthalpy is different for different bonds within the same molecule.
Detailed Explanation
Polyatomic molecules, such as methane (CH4), have multiple types of bonds. In such cases, the bond enthalpy will vary between different types of bonds. For example, the energy required to break the first carbon-hydrogen bond in methane may differ from breaking the second or third. This variability arises due to the influence of other nearby atoms (their electronic effects) and the structural environment within the molecule.
Examples & Analogies
If you think about a necklace made of different types of beads, each bead (or bond) might require a different amount of effort to remove based on how tightly it's strung on the string. The energy to remove each bead is akin to the different bond dissociation enthalpies for various bonds within a polyatomic molecule.
Mean Bond Enthalpy
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In such cases we use mean bond enthalpy of C – H bond. For example in CH4, ∆C–H H is calculated as: ∆C–H H = ¼ (∆a H) = ¼ (1665 kJ mol–1) = 416 kJ mol–1.
Detailed Explanation
Mean bond enthalpy is an average value for bond strengths in molecules that contain multiple identical bonds. For methane, the four C-H bonds are identical, but the energy required to break them sequentially is different. To simplify calculations, we can average these values to find a mean value for bond strength, which helps in predicting reaction energies and understanding molecular stability.
Examples & Analogies
Consider a classroom full of chairs arranged in rows. Each row can be viewed as a bond type. While the chairs in the front row might be difficult to move out for cleaning versus the last row that is easy to move, if you were to take an average of the effort required to move any chair in the classroom, you’d get a general idea of ‘average chair difficulty’ – this is similar to how mean bond enthalpy provides an averaged strength across identical bonds.
Key Concepts
-
Bond Enthalpy: Energy required to break a bond.
-
Bond Dissociation Enthalpy: Specific bond energy change.
-
Mean Bond Enthalpy: Average energy across similar bonds.
Examples & Applications
Bond Dissociation Enthalpy for H-H bond in H2: 435 kJ/mol.
Mean Bond Enthalpy of C-H bonds in methane is typically around 410 kJ/mol.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To break a bond, use energy's might, bond enthalpy’s the key, in reactions, that's right!
Stories
Imagine a magic chemist who could see the bonds breaking and forming—each bond needed a special spell (energy) to break it apart.
Memory Tools
B.E - Bonds Ended: Remember that breaking bonds requires energy!
Acronyms
B.E.S. - Bonds Energy Strength
Helps remember that the energy required relates to bond strength.
Flash Cards
Glossary
- Bond Enthalpy
The energy required to break a specific bond in a molecule.
- Bond Dissociation Enthalpy
The change in enthalpy when one mole of a bond in a molecule is broken.
- Mean Bond Enthalpy
The average energy required to break a particular type of bond across a range of similar molecules.
Reference links
Supplementary resources to enhance your learning experience.
