The Relationship between Cp and CV
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Heat Capacities
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning, class! Today, we're going to discuss two important concepts in thermodynamics: heat capacities at constant pressure (Cp) and constant volume (CV). Can anyone tell me what heat capacity refers to?

Is it the amount of heat required to raise the temperature of a substance?

Exactly! Heat capacity measures how much heat a substance can hold. Now, what's the difference when we say at constant pressure versus constant volume?

At constant volume, the volume doesn’t change, while at constant pressure, the pressure remains the same?

Great summary! Let's remember: Cp is used in processes where the substance can expand, allowing pressure to remain constant, while CV is used when the substance is constrained to a fixed volume.
Deriving the Relationship between Cp and CV
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

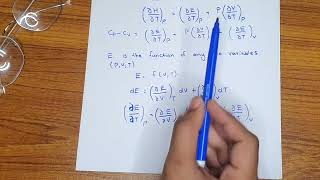
Now, let’s delve into how we can derive the relationship between these two heat capacities. The generalized formula for enthalpy is ΔH = ΔU + Δ(pV). Do you remember what ΔU represents?

It's the change in internal energy!

Correct! For an ideal gas, we can also consider Δ(pV) as RΔT. This leads us to the equation ΔH = ΔU + RΔT. Thus, if we differentiate at constant pressure, we relate it to Cp and CV.

So, does that mean we can calculate Cp using CV?

Yes! The final relationship we derive is Cp - CV = R. This tells us that the difference in heat capacities at constant pressure and volume is equal to the ideal gas constant R.
Applications of Cp and CV in Real-Life Scenarios
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we have established this vital relationship, why do you think it's important to know the difference between Cp and CV in real-world applications like engines or refrigerators?

It probably helps in designing systems to control heat transfer efficiently.

Exactly! Understanding these properties allows engineers to calculate heat requirements and efficiency in thermal systems. Could you think of an example of where this might be useful?

In car engines, right? The fuel combustion and the heat transfer to keep everything at the right temperature.

Spot on! Knowing how to balance these heat capacities helps ensure efficient engine performance.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Understanding the relationship between Cp and CV is essential for thermodynamic processes. This section explains how to derive the relationship through enthalpy and internal energy changes, with specific focus on ideal gases.
Detailed
In thermodynamics, Cp and CV represent the heat capacities at constant pressure and constant volume, respectively. For an ideal gas, the difference between these two heat capacities can be derived from enthalpy and internal energy changes. This relationship is given by the equation Cp - CV = R, where R is the ideal gas constant. This relationship is crucial in thermodynamic calculations involving changes in temperature, energy, and phase transitions. An understanding of this relationship allows scientists and engineers to predict how gases behave under different conditions, which is essential in chemical reactions and industrial applications.
Youtube Videos





Key Concepts
-
Heat Capacity: The amount of heat required to raise the temperature of a substance.
-
Cp and CV: Cp is used at constant pressure, while CV is used at constant volume.
-
Relationship: Cp - CV = R for ideal gases, illustrating the connection between heat capacities.
Examples & Applications
An ideal gas at constant pressure absorbs heat, causing its temperature to rise, demonstrating the application of Cp.
When a gas is compressed in a rigid container, the heat absorbed is related to CV.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Cp is for pressure, CV for volume, choose the right one to avoid the heat's gloom!
Stories
Imagine you’re in a kitchen, cooking under pressure (Cp) and sealed in a jar (CV). Which one lets you expand your flavor? That's the secret sauce!
Memory Tools
Remember: C equals capacity; P is pressure, V is volume. C(p) is for Pressure.
Acronyms
CP
Capacity under Pressure. CV
Flash Cards
Glossary
- Cp
Heat capacity at constant pressure.
- CV
Heat capacity at constant volume.
- Ideal Gas
A hypothetical gas that perfectly follows the ideal gas law.
- R
The ideal gas constant, which relates pressure, volume, temperature, and the number of moles.
Reference links
Supplementary resources to enhance your learning experience.
