Enthalpy change, ∆rH of a reaction – Reaction Enthalpy
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Reaction Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss reaction enthalpy, denoted as ∆rH. Can anyone tell me what they think ‘enthalpy change’ means?

Is it the heat change during a reaction?

Exactly! It's the heat absorbed or released during a reaction at constant pressure. We compute it as the difference in total enthalpies between products and reactants. Can someone give me the formula for that?

I think it’s ∆rH = (sum of enthalpies of products) - (sum of enthalpies of reactants).

Good job! Remember that ∆rH can be positive for endothermic reactions or negative for exothermic reactions. To remember this, think of the acronym 'PEACH': Positive for Endothermic, and AboVe zero for Heat.

What does Hess's Law have to do with this?

Great question! Hess's Law allows us to calculate ∆rH for reactions that cannot be directly measured. It states that the total enthalpy change is the sum of enthalpy changes for the steps. Always keep in mind, 'Hess says sum it up!'
Standard States and Their Importance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand enthalpy change, let’s discuss standard states. Who can tell me why they are important?

They provide a reference point for measuring enthalpy changes?

Exactly! Standard states define the pure and stable forms of reactants and products at 1 bar and usually 298 K. It's crucial for calculating the standard enthalpy of reactions, which we denote as ∆rH°.

Can you give an example of a reaction showing standard enthalpy?

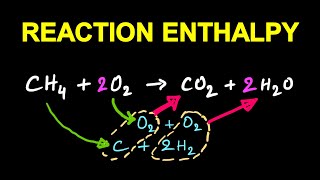
Sure! For the combustion of methane: CH₄ + 2O₂ → CO₂ + 2H₂O. The standard enthalpy change can be calculated and is crucial for energy assessments in combustion engines.

So it’s useful for real-world applications?

Absolutely! Understanding these enthalpy values aids in reaction predictions and industrial processes. Remember: Heating or cooling reaction setups requires knowing those enthalpy changes!
Using Hess's Law in Calculations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s see how we apply Hess's Law in practical calculations. What is the first step?

Set up the reactions individually that lead to the desired reaction?

Right again! By doing so, the enthalpy changes we have for those individual steps can be summed up. Let's take the combustion of carbon as an example.

What if we want to calculate the enthalpy change for that reaction?

You would use the known values for the formation of CO₂ and then apply Hess's Law. Remember the phrase, ‘Hess’s steps for reactions’ when completing these calculations!

Can we do that for any reaction?

If you can break it down into steps for which you have enthalpy values, then yes! Always visualize it as stacking up enthalpy values after each step.
Calculating Reaction Enthalpy with Given Data
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's practice! If we know the formation enthalpies of reactants and products, how do we calculate ∆rH?

We just use the formula you gave us earlier about summing them up?

Correct! And remember to keep all units consistent. Who can tell me why precision in these calculations matters?

Because small errors can lead to big discrepancies in real-world applications?

Exactly! That's why clear, consistent units and careful calculations are critical. Can anyone summarize the steps for us?

Calculate reactants' and products' enthalpies separately, then apply Hess’s Law to get the overall change!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the enthalpy change associated with chemical reactions is defined as the difference in the total enthalpies of products and reactants. Standard states are presented, alongside the application of Hess's law of constant heat summation, which allows for the calculation of enthalpy changes for various reactions.
Detailed
Detailed Summary
Enthalpy change (denoted as ∆rH) is an essential thermodynamic quantity that occurs during chemical reactions. It can be calculated as the difference between the sum of the enthalpies of the products and the sum of the enthalpies of the reactants in a chemical reaction. This is mathematically represented as:
Understanding standard conditions is crucial, as it allows us to define the standard enthalpy of reaction (∆rH°), which is the enthalpy change when all reactants and products are in their standard states (pure forms at a specific temperature and pressure, generally 1 bar and 298 K).
Hess's Law plays a pivotal role in thermodynamics, affirming that the total enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps, regardless of the actual pathway taken. This law supports calculating enthalpy changes for reactions that cannot be measured directly.
Reactants and products in chemical reactions are denoted as follows:
Reactants → Products
The section emphasizes that knowing the enthalpy changes for reactions is vital for various applications, such as determining heating or cooling requirements in industrial processes, and understanding temperature dependencies in equilibrium constants.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Reaction Enthalpy
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In a chemical reaction, reactants are converted into products and is represented by,
Reactants → Products
The enthalpy change accompanying a reaction is called the reaction enthalpy. The enthalpy change of a chemical reaction, is given by the symbol ∆rH.
∆rH = (sum of enthalpies of products) – (sum of enthalpies of reactants)
Detailed Explanation
The reaction enthalpy (∆rH) represents the heat absorbed or released during a chemical reaction at constant pressure. To calculate it, you take the enthalpy of the products and subtract the enthalpy of the reactants. This gives a numerical value that tells us if the reaction is exothermic (releases heat, ∆rH is negative) or endothermic (absorbs heat, ∆rH is positive).
Examples & Analogies
Think of baking a cake. When you mix ingredients (the reactants), and heat them in the oven, you obtain a cake (the product). The energy you need to bake (heat absorbed) is analogous to endothermic reactions, while the heat released from burning wood in a fireplace (exothermic reaction) can warm your house.
Calculating Reaction Enthalpy
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
∆rH = (sum of enthalpies of products) – (sum of enthalpies of reactants)
Here, the symbol ∑ is used for summation and ai and bi are the stoichiometric coefficients of the products and reactants respectively in the balanced chemical equation.
Detailed Explanation
In this formula, the summation sign (∑) emphasizes that you must account for each product and reactant in the balanced equation according to their coefficients. For example, if a reaction produces water (H2O) and carbon dioxide (CO2) in a balanced equation like 2 H2 + O2 → 2 H2O, the enthalpy of the products would include 2 times the enthalpy of H2O.
Examples & Analogies
Imagine you are building a LEGO set. The enthalpy change here is like counting all the pieces you used to find out how much was added (products) versus how many you started with (reactants). If more pieces are needed to complete the set (endothermic), that's like needing more energy; if you're using pieces to break something down (exothermic), it’s like releasing energy.
Importance of Reaction Enthalpy
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Enthalpy change is a very useful quantity. Knowledge of this quantity is required when one needs to plan the heating or cooling required to maintain an industrial chemical reaction at constant temperature. It is also required to calculate temperature dependence of equilibrium constant.
Detailed Explanation
Understanding reaction enthalpy is crucial for controlling chemical processes, especially in industrial settings. It helps engineers decide how much energy is needed to keep a reaction going optimally. Additionally, knowing how enthalpy changes with temperature allows for calculations related to equilibrium states of reactions which are essential for maximizing product yield.
Examples & Analogies
Consider a chef preparing a dish that needs to be cooked at a specific temperature. Knowing how much heat (energy) is required allows the chef to adjust the oven temperature accordingly. Similarly, in chemical reactions, maintaining the right enthalpy ensures the reaction proceeds efficiently.
Key Concepts
-
Enthalpy Change (∆rH): The heat change associated with a reaction, calculated as the difference in enthalpies between products and reactants.
-
Standard State: A reference condition of a substance under 1 bar and typically 298 K, used for calculating standard enthalpy changes.
-
Hess's Law: A principle that allows the calculation of reaction enthalpy using the sum of enthalpies of individual steps.
Examples & Applications
The combustion of methane can be shown as: CH₄ + 2O₂ → CO₂ + 2H₂O with ∆rH being negative, indicating an exothermic reaction.
Standard enthalpy of formation of water is defined under standard conditions, which helps to compute reaction enthalpies effectively.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Heat absorbed from the air, endotherm reactions show flair!
Stories
Once in a lab, chemists mixed and watched their reactions go from bright to dark—heat flowing in, making the environment feel like a cozy embrace, that's how endotherms thrive.
Memory Tools
‘PERE’ - Products Enthalpy - Reactants Enthalpy for enthalpy changes.
Acronyms
HESS
Heat Enthalpy Summation State.
Flash Cards
Glossary
- Enthalpy Change (∆rH)
The heat content change of a system during a chemical reaction at constant pressure.
- Standard State
A reference condition for a substance, typically defined as the pure form at 1 bar and usually 298 K.
- Hess's Law
A principle stating that the total enthalpy change during a chemical reaction is the same, regardless of the number of steps in the reaction.
- Enthalpy of Reaction
The energy change associated with a reaction, measured either directly or calculated using standard enthalpies.
- Exothermic Reaction
A reaction that releases heat, resulting in a negative enthalpy change (∆rH < 0).
- Endothermic Reaction
A reaction that absorbs heat, resulting in a positive enthalpy change (∆rH > 0).
Reference links
Supplementary resources to enhance your learning experience.
